2022 wholesale price Medical Kit - C-creactive protein CRP rapid test kit – Baysen
2022 wholesale price Medical Kit - C-creactive protein CRP rapid test kit – Baysen Detail:
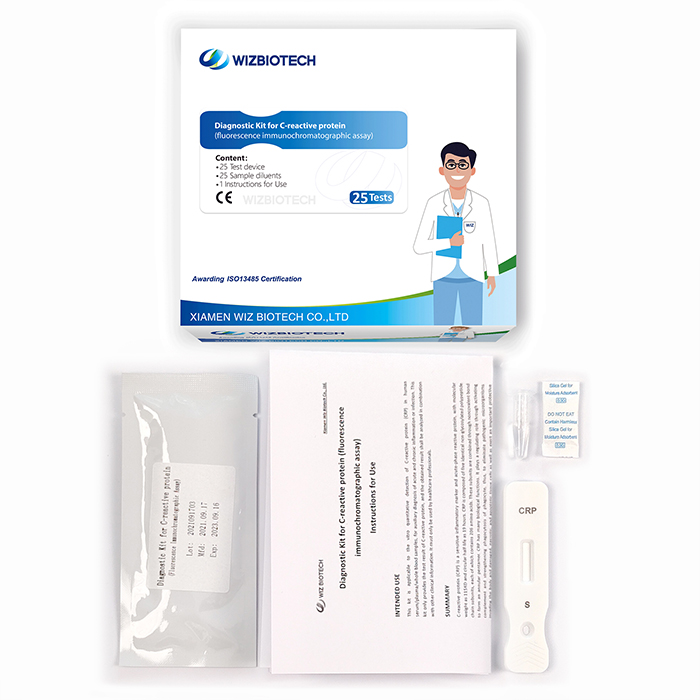
Diagnostic Kit for C-reactive Protein
Methodology:Fluorescence Immunochromatographic Assay
Production information
| Model Number | CRP | Packing | 25 Tests/ kit, 30kits/CTN |
| Name | Diagnostic Kit for
C-reactive Protein |
Instrument classification | Class I |
| Features | High sensitivity, Easy opeation | Certificate | CE/ ISO13485 |
| Accuracy | > 99% | Shelf life | Two Years |
| Methodology | Fluorescence Immunochromatographic Assay | OEM/ODM service | Avaliable |
INTENDED USE
This kit is applicable to the vitro quantitative detection of C-reactive protein (CRP) in human serum/plasma/whole blood samples, for auxiliary diagnosis of acute and chronic inflammation or infection. This kit only provides the test result of C-reactive protein, and the obtained result shall be analyzed in combination with other clinical information.
Test procedure
| 1 | Before using the reagent,read the package insert carefully and familiarize yourself with the operating procedures. |
| 2 | Select standard test mode of WIZ-A101 portable immune analyzer |
| 3 | Open the aluminum foil bag package of reagent and take out the test device. |
| 4 | Horizontally insert the test device into the slot of immune analyzer. |
| 5 | On home page of operation interface of immune analyzer, click “Standard” to enter test interface. |
| 6 | Click “QC Scan” to scan the QR code on inner side of the kit; input kit related parameters into instrument and select sample type. Note: Each batch number of the kit shall be scanned for one time. If the batch number has been scanned, then skip this step. |
| 7 | Check the consistency of “Product Name”, “Batch Number” etc. on test interface with information on the kit label. |
| 8 | Take out sample diluent upon consistent information, add 10μL serum/plasma/whole blood sample, and thoroughly mix them; |
| 9 | Add 80µL aforesaid thoroughly mixed solution into well of test device; |
| 10 | After complete sample addition, click “Timing” and remaining test time will be automatically displayed on the interface. |
| 11 | Immune analyzer will automatically complete test and analysis when test time is reached. |
| 12 | After test by immune analyzer is completed, test result will be displayed on test interface or can be viewedthrough “History” on home page of operation interface. |
Note: each sample shall be pipetted by clean disposable pipette to avoid cross contamination.
Product detail pictures:


Related Product Guide:
Prostate Cancer Screening Can Save Lives, But There Is a Hidden Cost | Calprotectin Elisa Kit
Weekly Dose: Keytruda may be a miracle cancer drug, but can those who need it afford it? | Psa Test Cost
No matter new customer or outdated client, We believe in extensive phrase and trusted relationship for 2022 wholesale price Medical Kit - C-creactive protein CRP rapid test kit – Baysen , The product will supply to all over the world, such as: Bandung, Gambia, Armenia, Our products are sold to the Middle East, Southeast Asia, Africa, Europe, America and other regions, and are favorably appraised by clients. To benefit from our strong OEM/ODM capabilities and considerate services, please contact us today. We will sincerely create and share success with all clients.
The company account manager has a wealth of industry knowledge and experience, he could provide appropriate program according our needs and speak English fluently.