Diagnostic Kit for Gastrin-17 ( Fluorescence Immuno Assay)
FOB Brochure
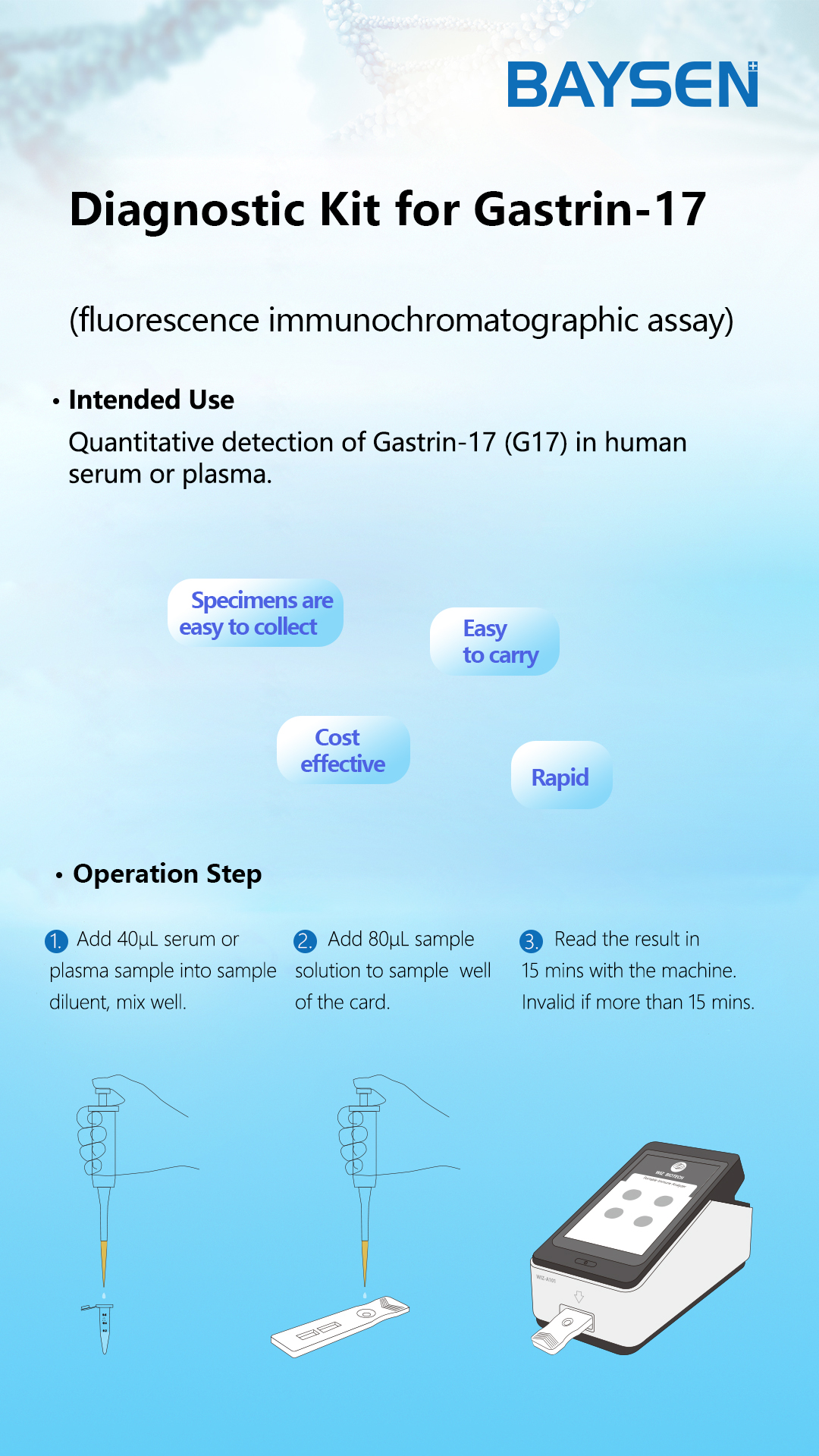

PRINCIPLE AND PROCEDURE OF FOB TEST
Princple:
The strip has anti-FOB coating antibody on test region, which is fastened to membrane chromatography in advance. Lable pad is coated by fluorescence labeled anti-FOB antibody in advance. When testing positive sample, the FOB in sample can be mixed with fluorescence labeled anti-FOB antibody, and form immune mixture. As the mixture is allowed to migrate along the test strip, the FOB conjugate complex is captured by anti-FOB coating antibody on the membrane and forms complex. The fluorescence intensity is positively correlated with the FOB content. The FOB in sample can be detected by fluorescence immunoassay analyzer.
Test Procedure:
1.Lay aside all reagents and samples to room temperature.
2.Open the Portable Immune Analyzer(WIZ-A101), enter the account password login according to the operation method of the instrument, and enter the detection interface.
3.Scan the dentification code to confirm the test item.
4.Take out the test card from the foil bag.
5.Insert the test card into the card slot, scan the QR code, and determine the test item.
6.Remove the cap from the sample tube and discard the first two drops diluted sample, add 3 drops (about 100uL) no bubble diluted sample verticaly and slowly into sample well of the card with provided dispette.
7.Click the "standard test" button, after 15 minutes, the instrument will automatically detect the test card, it can read the results from the display screen of the instrument, and record/print the test results.
8.Refer to the instruction of Portable Immune Analyzer(WIZ-A101).

About Us

Xiamen Baysen Medical Tech limited is a high biological enterprise which devotes itself to filed of fast diagnostic reagent and integrates research and development, production and sales into a whole. There are many advanced research staffs and sales managers in the company, all of them are have rich working experience in china and international biopharmaceutical enterprise.
Certificate display