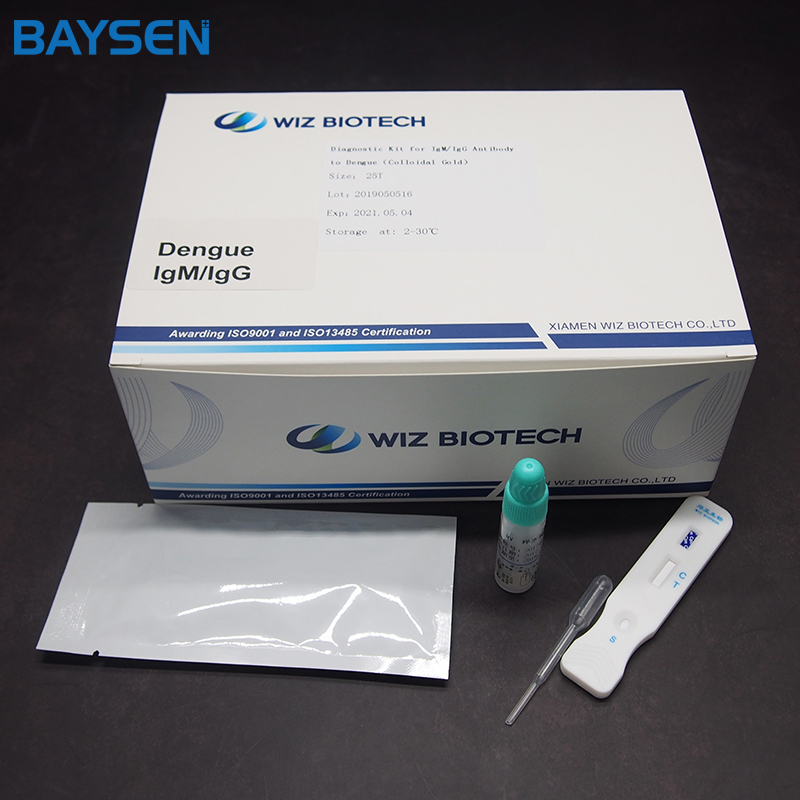
China OEM Diagnostic Kit For Human Chorionic Gonadotropin - OEM China China Lansionbio CE ISO Approved Amh One Step Rapid Test Kit – Baysen
China OEM Diagnostic Kit For Human Chorionic Gonadotropin - OEM China China Lansionbio CE ISO Approved Amh One Step Rapid Test Kit – Baysen Detail:
We believe in: Innovation is our soul and spirit. Excellent is our life. Shopper need to have is our God for OEM China China Lansionbio CE ISO Approved Amh One Step Rapid Test Kit, Our really specialized process eliminates the component failure and offers our shoppers unvarying top quality, allowing us to control cost, plan capacity and maintain consistent on time delivery.
We believe in: Innovation is our soul and spirit. Excellent is our life. Shopper need to have is our God for China Rapid Test Kit, Clinical Diagnostic Test Kit, Our faith is to be honest first, so we just supply high quality goods to our customers. Genuinely hope that we can be business partners. We believe that we can establish long time business relationship with each other. You can contact us freely for more information and pricelist of our products and solutions !
FOB Brochure


PRINCIPLE AND PROCEDURE OF FOB TEST
Princple:
The strip has anti-FOB coating antibody on test region, which is fastened to membrane chromatography in advance. Lable pad is coated by fluorescence labeled anti-FOB antibody in advance. When testing positive sample, the FOB in sample can be mixed with fluorescence labeled anti-FOB antibody, and form immune mixture. As the mixture is allowed to migrate along the test strip, the FOB conjugate complex is captured by anti-FOB coating antibody on the membrane and forms complex. The fluorescence intensity is positively correlated with the FOB content. The FOB in sample can be detected by fluorescence immunoassay analyzer.
Test Procedure:
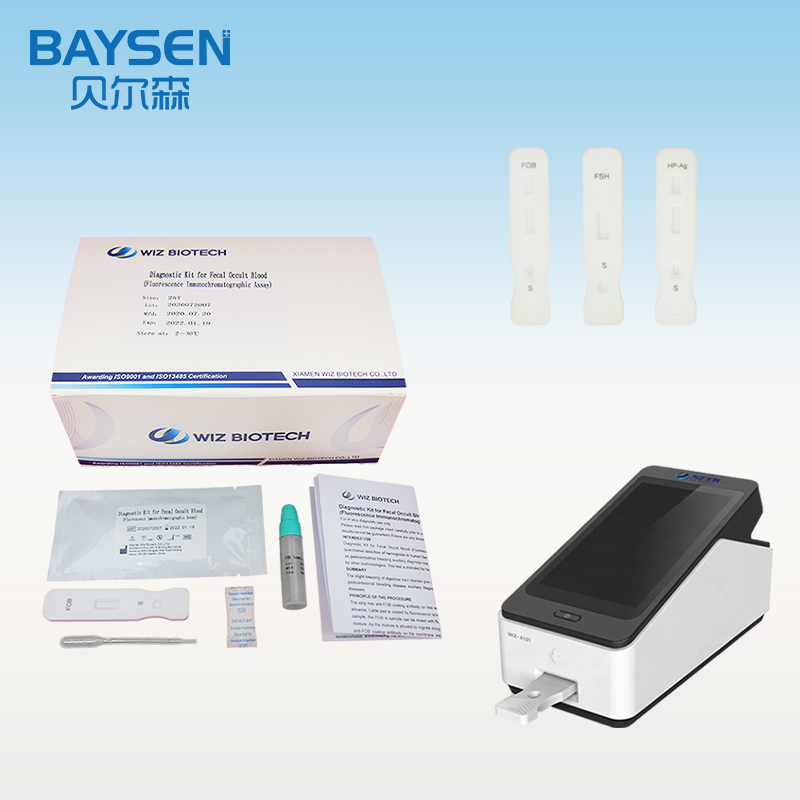
1.Lay aside all reagents and samples to room temperature.
2.Open the Portable Immune Analyzer(WIZ-A101), enter the account password login according to the operation method of the instrument, and enter the detection interface.
3.Scan the dentification code to confirm the test item.
4.Take out the test card from the foil bag.
5.Insert the test card into the card slot, scan the QR code, and determine the test item.
6.Remove the cap from the sample tube and discard the first two drops diluted sample, add 3 drops (about 100uL) no bubble diluted sample verticaly and slowly into sample well of the card with provided dispette.
7.Click the “standard test” button, after 15 minutes, the instrument will automatically detect the test card, it can read the results from the display screen of the instrument, and record/print the test results.
8.Refer to the instruction of Portable Immune Analyzer(WIZ-A101).

You May like
SARS-CoV-2 Antigen Rapid Test(Colloidal Gold)
WIZ-A101 Portable Immune Analyzer
Diagnostic Kit for Anti-Mullerian Hormone (Fluorescence Immunochromatographic Assay)
About Us

Xiamen Baysen Medical Tech limited is a high biological enterprise which devotes itself to filed of fast diagnostic reagent and integrates research and development, production and sales into a whole. There are many advanced research staffs and sales managers in the company, all of them are have rich working experience in china and international biopharmaceutical enterprise.
Certificate display

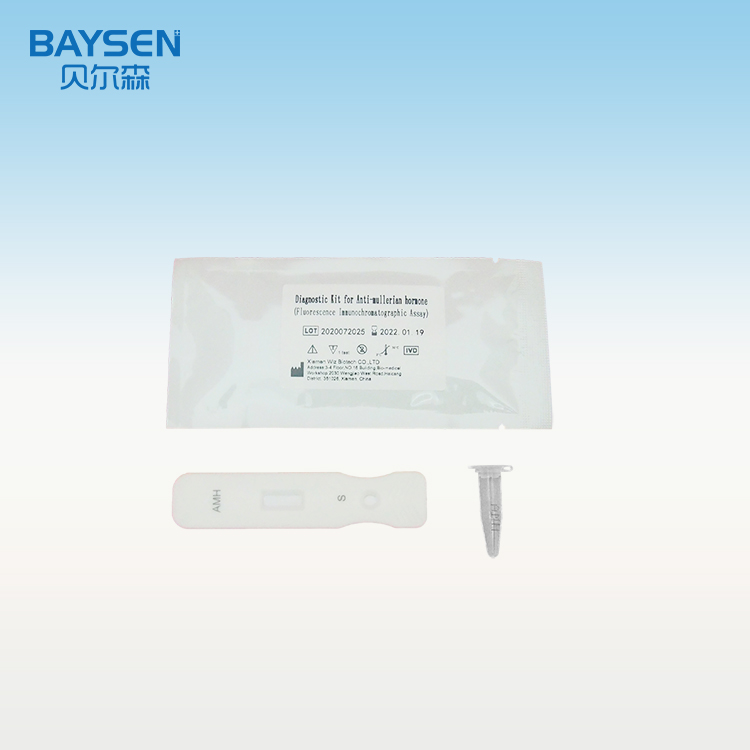
Product detail pictures:


Related Product Guide:
Conjoint Professor Peter Gibson / Staff Profile / The University of Newcastle, Australia | Psa Test Cost
Prostate Cancer: Aging with cancer as side-effects continue to linger | Calprotectin Elisa Kit
We will make just about every exertion for being excellent and perfect, and speed up our actions for standing during the rank of worldwide top-grade and high-tech enterprises for China OEM Diagnostic Kit For Human Chorionic Gonadotropin - OEM China China Lansionbio CE ISO Approved Amh One Step Rapid Test Kit – Baysen , The product will supply to all over the world, such as: Zurich, Detroit, Cancun, When you are keen on any of our goods following you view our product list, be sure to feel free to make contact with us for inquiries. You'll be able to send us emails and get in touch with us for consultation and we shall respond to you as soon as we're able to. If it's convenient, you could find out our address in our web site and come to our enterprise. or additional information of our products by yourself. We're generally ready to build lengthy and steady co-operation relations with any possible shoppers within the associated fields.
The company keeps to the operation concept "scientific management, high quality and efficiency primacy, customer supreme", we have always maintained business cooperation. Work with you,we feel easy!