Diagnostic Kit(Colloidal Gold)for Calprotectin
Diagnostic Kit(Colloidal Gold)for Calprotectin
For in vitro diagnostic use only
Please read this package insert carefully prior to use and strictly follow the instructions. Reliability of assay results cannot be guaranteed if there are any deviations from the instructions in this package insert.
INTENDED USE
Diagnostic Kit for Calprotectin(cal) is is a colloidal gold immunochromatographic assay for the semiquantitative determination of cal from human faeces, which has important accessory diagnostic value for inflammatory bowel disease. This test is a screening reagent. All positive sample must be confirmed by other methodologies. This test is intended for healthcare professional use only. Meanwhile, this test is used for IVD, extra instruments are not needed.
SUMMARY
Cal is a heterodimer, which is composed of MRP 8 and MRP 14. It exists in neutrophils cytoplasm and expressed on mononuclear cell membranes. Cal is acute phase proteins, it has a well stable phase about one week in human faeces, it is determined to be a inflammatory bowel disease marker. The kit is a simple, visual semiqualitative test that detects cal in human faeces, it has high detection sensitivity and strong specificity. The test based on high specificit double antibodies sandwich reaction principle and gold immunochromatographic assay analysis technics, it can give a result within 15 minutes.
PRINCIPLE OF THE PROCEDURE
The strip has anti cal coating McAb on test region and goat anti-rabbit IgG antibody on control region, which is fastened to membrane chromatography in advance. Lable pad is coated by colloidal gold labeled anti cal McAb and colloidal gold labeled rabbit IgG antibody in advance. When testing positive sample, the cal in sample comined with colloidal gold labeled anti cal McAb, and form immune complex, as it is allowed to migrate along the test strip, the cal conjugate complex is captured by anti cal coating McAb on the membrane and form “anti cal coating McAb-cal-colloidal gold labeled anti cal McAb” complex, a colored test band appeared on test region. The color intensity is positively correlated with the cal content. A negative sample does not produce a test band due to the absence of colloidal gold conjugate cal complex. No matter cal is present in sample or not, there is a red stripe appear on reference region and quality control region, which is regarded as quality internal enterprise standards.
REAGENTS AND MATERIALS SUPPLIED
25T package components:
.Test card individually foil pouched with a desiccant
.Sample diluents: the ingredients is 20mM pH7.4PBS
.Dispette
.Package insert
MATERIALS REQUIRED BUT NOT PROVIDED
Sample collection container, timer
SAMPLE COLLECTION AND STORAGE
Use a disposable clean container to collect fresh faeces sample, and tested immediately. If can not be tested immediately, please stored at 2-8°C for 12hours or bellow -15°C for 4 months.
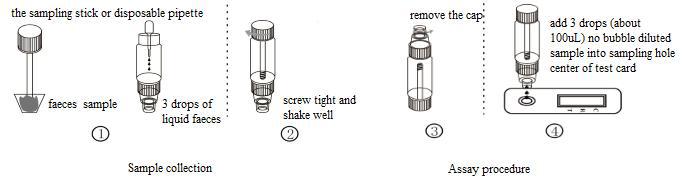
ASSAY PROCEDURE
1.Take out the sampling stick, inserted into the faeces sample, then put the sampling stick back, screw tight and shake well, repeat the action 3 times. Or using the sampling stickpicked about 50mg faeces sample, and put in a faeces sample tube containing sample dilution, and screw tightly.
2.Use disposable pipette sampling take the thinner faeces sample from the diarrhea patient, then add 3 drops (about 100uL) to the fecal sampling tube and shake well, put aside.
3.Take out the test card from the foil bag, put it on the level table and mark it.
4.Remove the cap from the sample tube and discard the first two drops diluted sample, add 3 drops (about 100uL) no bubble diluted sample verticaly and slowly into sample well of the card with provided dispette, start timing.
5.The result should be read within 10-15 minutes, and it is invalid after 15 minutes.
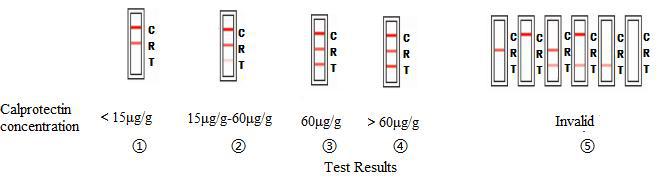
TEST RESULTS AND INTERPRETATION
| Test results | Interpretation | |
| ① | Red reference band and red control bandappear on R region and C region, no redtest band on T region. | It means the content of human faecescalprotectin is under15μg/g, which is anormal level. |
| ② | Red reference band and red control bandappear on R region and C region, and thecolor of red reference band is darker thanred test band. | The content of human faeces calprotectin isbetween 15μg/g and 60μg/g. That may bein the normal level, or there may be a risk ofIrritable Bowel Syndrome. |
| ③ | Red reference band and red control bandappear on R region and C region, and thecolor of red reference band is same withred test band. | The content of human faeces calprotectin is60μg/g, and there is existential risk ofinflammatory bowel disease. |
| ④ | Red reference band and red control bandappear on R region and C region, and thecolor of red test band is darker than redreference band. | It indicates the content of human faecescalprotectin is more than 60μg/g, and thereis existential risk of inflammatory boweldisease. |
| ⑤ | If red reference band and red control bandis not seen or just seen only one,the test isconsidered invalid. | Repeat the test using a new test card. |
STORAGE AND STABILITY
The kit is 24 months shelf-life from the date of manufacture. Store the unused kits at 2-30°C. Do not open the sealed pouch until you are ready to perform a test.
WARNINGS AND PRECAUTIONS
1.The kit should be sealed and protected against moisture1.
2.Do not use sample which is placed too long or repeated freezing and thawing to test
3.Fecal samples is excessive or thickness can make the diluted samples foul test card, please centrifuge the diluted sample and take the supernatant for testing.
4.Misoperation, excessive or little sample can lead to result deviations.
LIMITATION
1.This test result is only for clinical reference, should not serve as the only basis for clinical diagnosis and treatment,the patients clinical management should be comprehensive consideration combined with its symptoms, medical history,other laboratory examination, treatment response,epidemiology and other information2 .
2.This reagent is only used for fecal tests. It may not obtain accurate result when used for other samples such as saliva and urine and etc.
REFERENCES
[1] The national clinical test procedures(the third edition,2006).The ministry health department.
[2] Measures for the administration of in vitro diagnostic reagents registration. China Food and Drug Administration ,No. 5 order, 2014-07-30.
Key to symbols used:
 |
In Vitro Diagnostic Medical Device |
 |
Manufacturer |
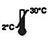 |
Store at 2-30℃ |
 |
Expiration Date |
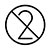 |
Do Not Reuse |
 |
CAUTION |
 |
Consult Instructions For Use |
Xiamen Wiz Biotech CO.,LTD
Address:3-4 Floor,NO.16 Building,Bio-medical Workshop,2030 Wengjiao West Road,Haicang District,361026,Xiamen,China
Tel:+86-592-6808278
Fax:+86-592-6808279
















