Factory making Chlamydia Home Test - Quantative Rapid Detection Test for Luteinizing Hormone (LH) – Baysen
Factory making Chlamydia Home Test - Quantative Rapid Detection Test for Luteinizing Hormone (LH) – Baysen Detail:
Product information
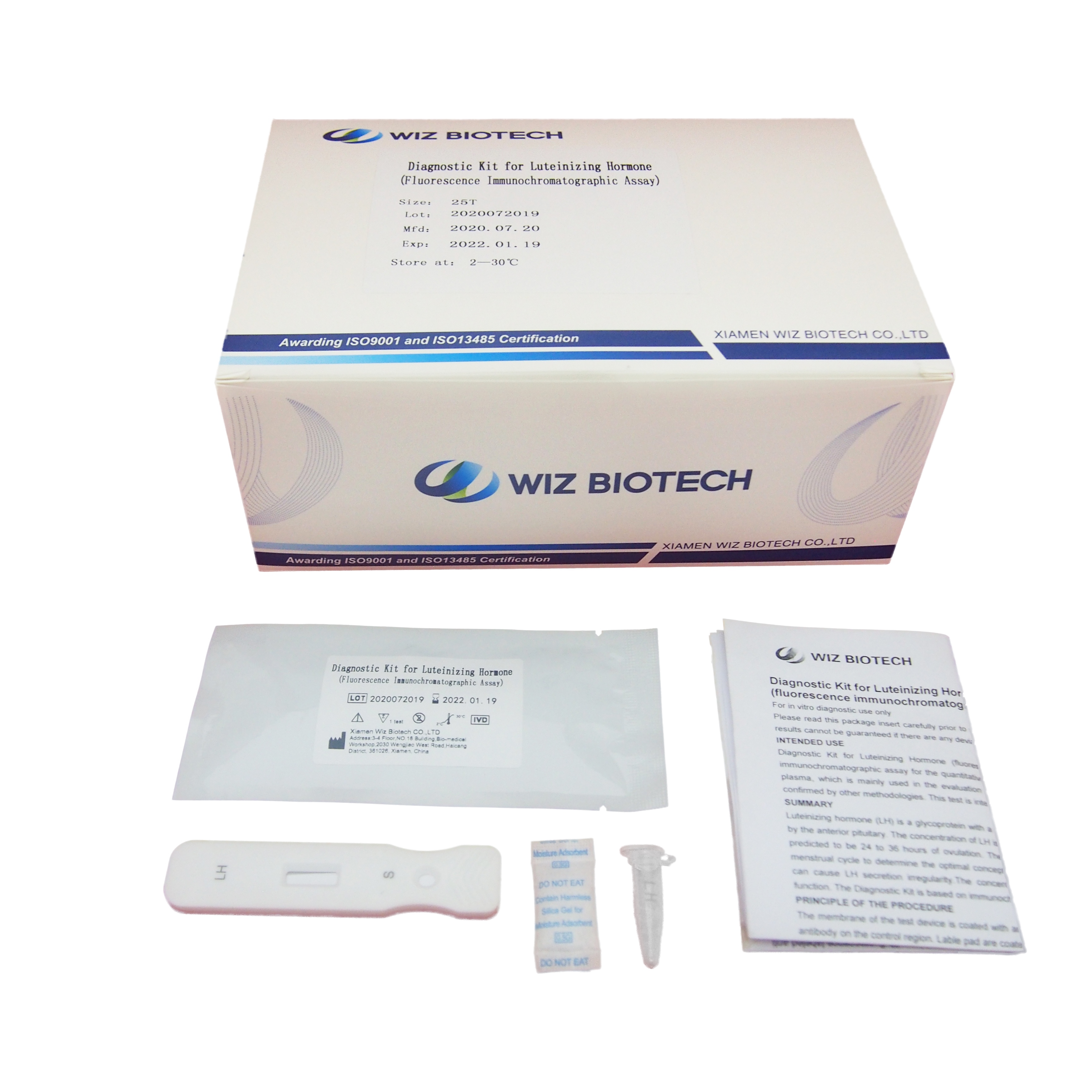
Name: Diagnostic Kit for Luteinizing Hormone(fluorescence immunochromatographic assay)
Summary :
Luteinizing hormone (LH) is a glycoprotein with a molecular weight of about 30,000 Dalton, which is produced by the anterior pituitary. The concentration of LH is closely related to ovulation of ovaries, and the peak of LH is predicted to be 24 to 36 hours of ovulation. Therefore, the peak value of LH can be monitored during the menstrual cycle to determine the optimal conception time. Abnormal endocrine function in the pituitary gland can cause LH secretion irregularity.The concentration of LH can be used to evaluate pituitary endocrine function. The Diagnostic Kit is based on immunochromatography and can give a result within 15 minutes
| Model Number | LH | Packing | 25 Tests/ kit,20kits/CTN |
| Name |
Diagnostic Kit for Luteinizing Hormone (fluorescence immunochromatographic assay) |
Instrument classification | Class II |
| Features | High sensitivity, Easy opeation | Certificate | CE/ ISO13485 |
| Accuracy | > 99% | Shelf life | Two Years |
| Type | Pathological Analysis Equipments | Technology | Quantitative kit |
More related products
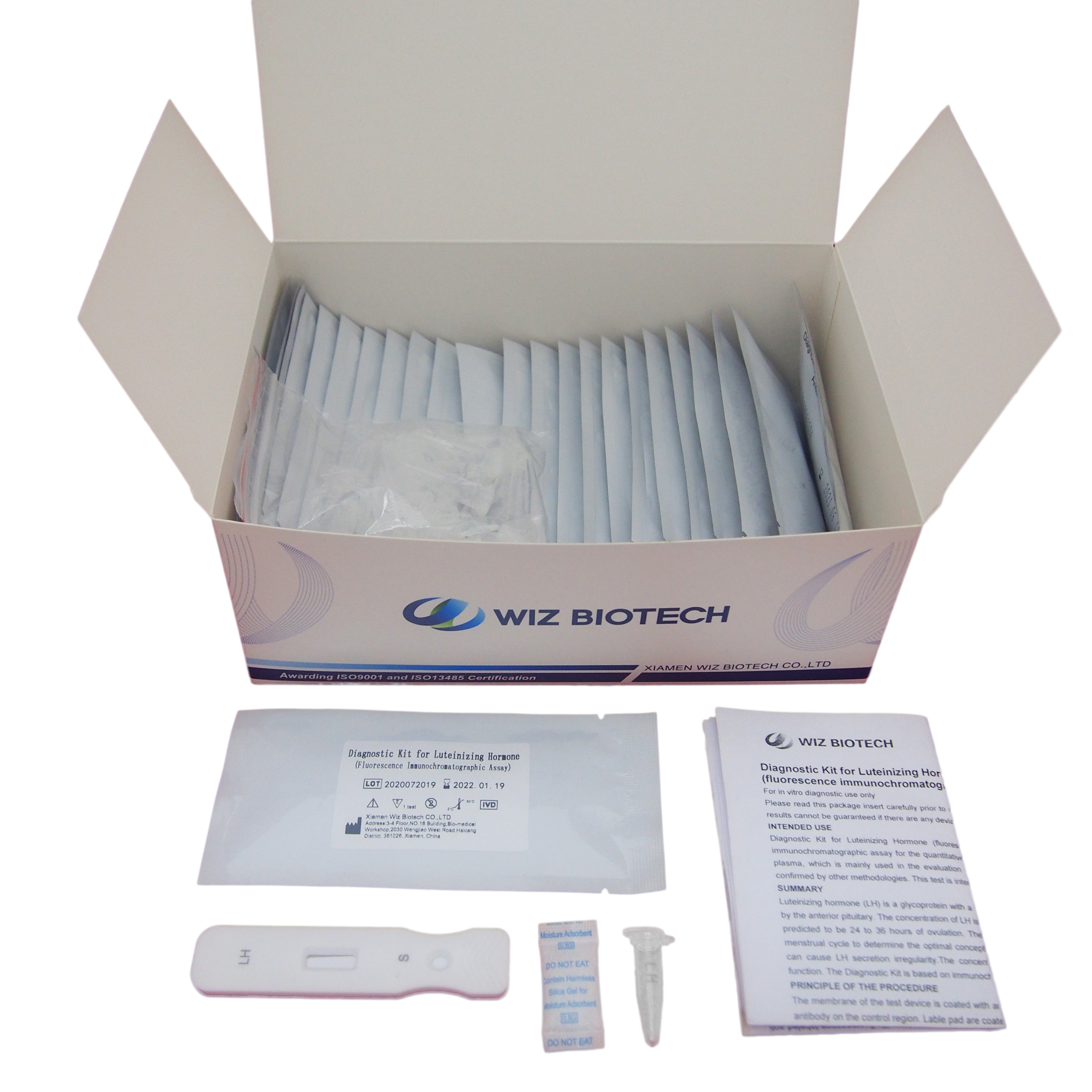
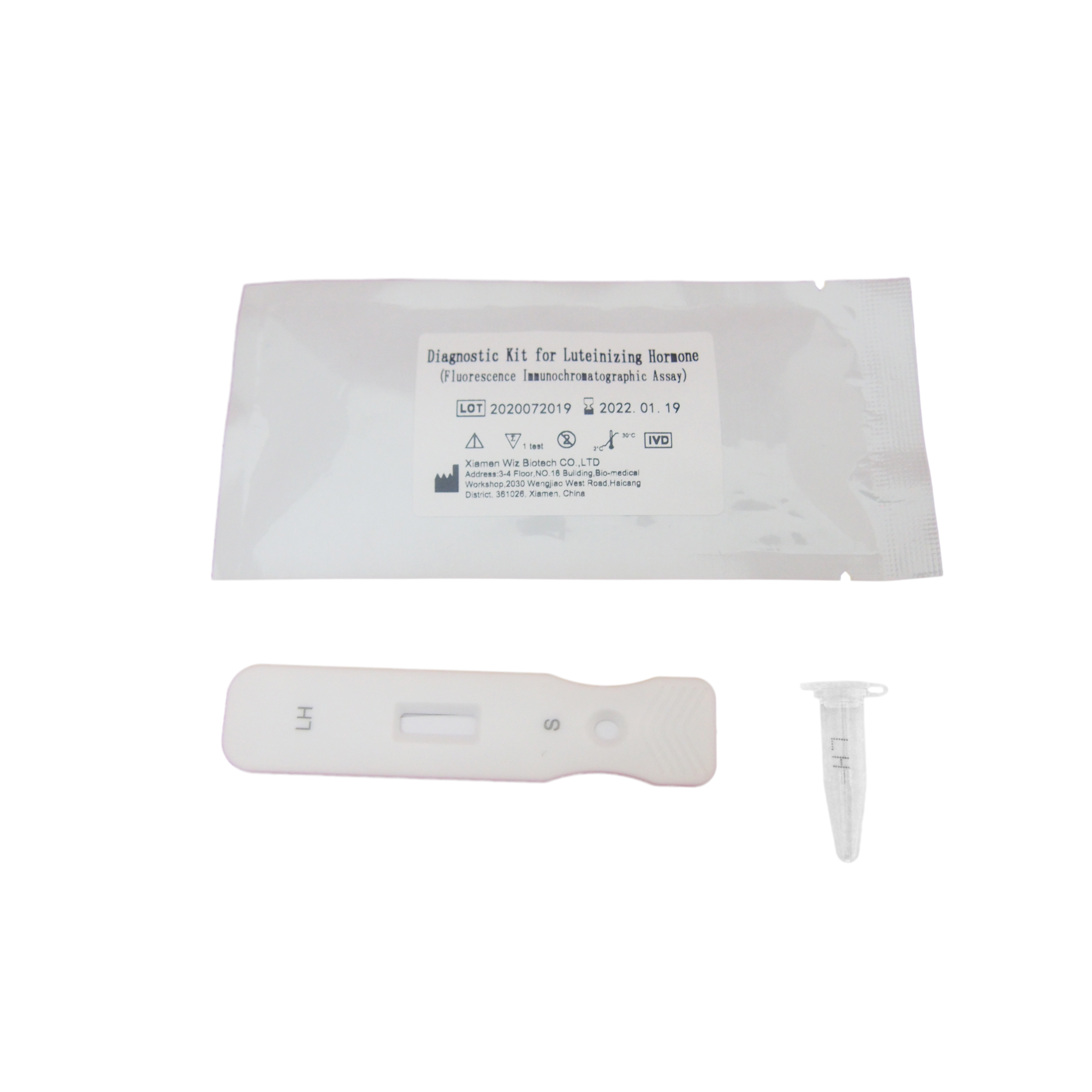
Product detail pictures:



Related Product Guide:
Is genetic testing sophisticated enough to make PSA screening viable for mainstream use? | Psa Test Cost
MDxHealth (R): SelectMDx Cost-effective in Four European Countries Brussels Stock Exchange:MDXH | Psa Test Cost
Being supported by an innovative and experienced IT team, we could present technical support on pre-sales & after-sales service for Factory making Chlamydia Home Test - Quantative Rapid Detection Test for Luteinizing Hormone (LH) – Baysen , The product will supply to all over the world, such as: India, Norway, Naples, We seriously promise that we provide all the customers with the best quality products, the most competitive prices and the most prompt delivery. We hope to win a resplendent future for customers and ourselves.
Hope that the company could stick to the enterprise spirit of "Quality, Efficiency, Innovation and Integrity", it will be better and better in the future.