Good Quality Semi-auto Automated Biochemistry Analyzer - Diagnostic Kit(Colloidal Gold)for Human Chorionic Gonadotrophin – Baysen
Good Quality Semi-auto Automated Biochemistry Analyzer - Diagnostic Kit(Colloidal Gold)for Human Chorionic Gonadotrophin – Baysen Detail:
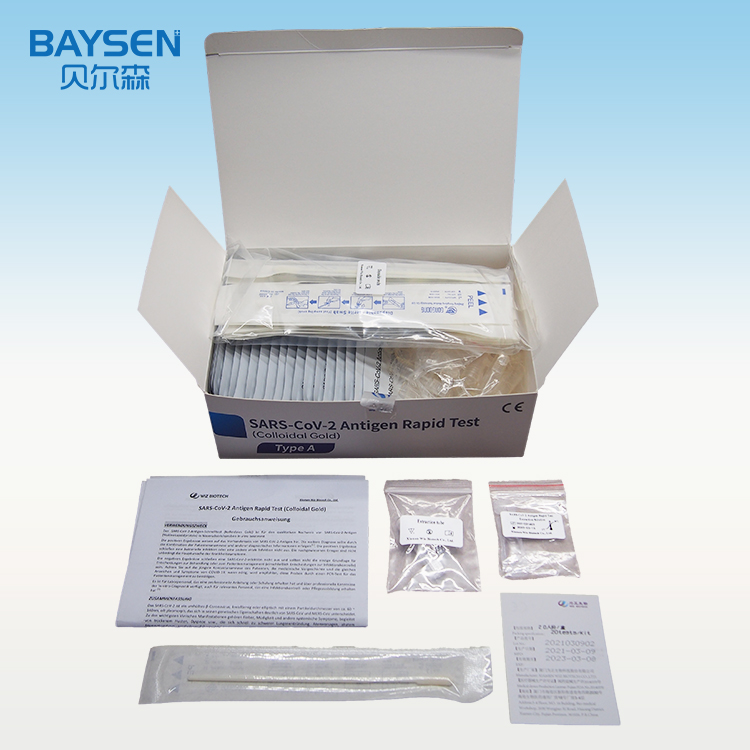
Diagnostic Kit(Colloidal Gold)for Human Chorionic Gonadotrophin
For in vitro diagnostic use only
Please read this package insert carefully prior to use and strictly follow the instructions. Reliability of assay results cannot be guaranteed if there are any deviations from the instructions in this package insert.
INTENDED USE
Diagnostic Kit(Colloidal Gold)for Human Chorionic Gonadotrophin is a colloidal gold immunochromatographic assay for the qualitative detection of human chorionic gonadotropin (HCG) levels in human serum and urine, it is used for early pregnancy diagnosis This test is a screening reagent. All positive sample must be confirmed by other methodologies. This test is intended for healthcare professional use only.
PACKAGE SIZE
1 kit /box, 10 kits /box, 25 kits,/box, 50 kits /box.
SUMMARY
HCG is a glycoprotein hormone secreted by the developing placenta after egg fertilization. HCG levels can be rapidly elevated in serum or urine as early as 1 to 2.5 weeks during pregnancy, and reach a peak in 8 week, than fall to medium level in 4 month, and maintained the level until the end of the pregnancy[1]. The Kit is a simple, visual qualitative test that detects HCG antigen in human serum or urine. The Diagnostic Kit is based on immunochromatography and can give a result within 15 minutes.
ASSAY PROCEDURE
1.Take out the test card from the foil bag, put it on the level table and mark it.
2.Discard the first two drops sample, add 3 drops (about 100μL) no bubble sample verticaly and slowly into sample well of the card with provided dispette, start timing.
3.The result should be read within 10-15 minutes, and it is invalid after 15 minutes.
Product detail pictures:

Related Product Guide:
MDxHealth (R): SelectMDx Cost-effective in Four European Countries Brussels Stock Exchange:MDXH | Psa Test Cost
10 health checks you can get on Medicare in Australia | Calprotectin Elisa Kit
We will make just about every exertion for being excellent and perfect, and speed up our actions for standing during the rank of worldwide top-grade and high-tech enterprises for Good Quality Semi-auto Automated Biochemistry Analyzer - Diagnostic Kit(Colloidal Gold)for Human Chorionic Gonadotrophin – Baysen , The product will supply to all over the world, such as: India, El Salvador, moldova, Many years of work experience, we have realized the importance of providing good quality products and the best before-sales and after-sales services. Most problems between suppliers and clients are due to poor communication. Culturally, suppliers can be reluctant to question things they do not understand. We break down those barriers to ensure you get what you want to the level you expect, when you want it. faster delivery time and the product you want is our Criterion .
Reasonable price, good attitude of consultation, finally we achieve a win-win situation,a happy cooperation!