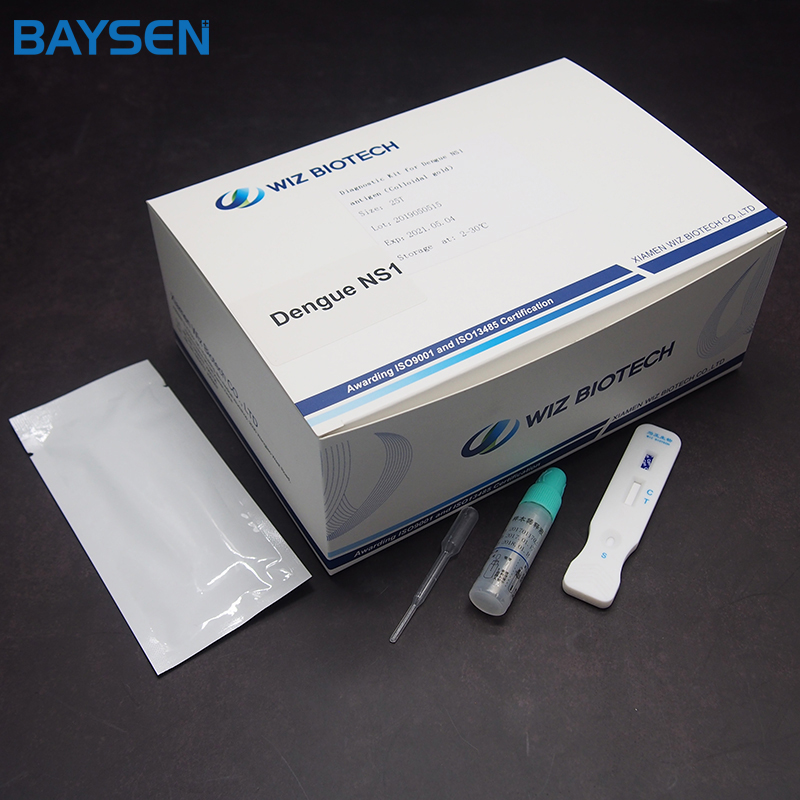
Manufactur standard Fob Psa Cea Afp Test Kit - Diagnostic Kit(Colloidal Gold)for Human Chorionic Gonadotrophin – Baysen
Manufactur standard Fob Psa Cea Afp Test Kit - Diagnostic Kit(Colloidal Gold)for Human Chorionic Gonadotrophin – Baysen Detail:
Diagnostic Kit(Colloidal Gold)for Human Chorionic Gonadotrophin
For in vitro diagnostic use only
Please read this package insert carefully prior to use and strictly follow the instructions. Reliability of assay results cannot be guaranteed if there are any deviations from the instructions in this package insert.
INTENDED USE
Diagnostic Kit(Colloidal Gold)for Human Chorionic Gonadotrophin is a colloidal gold immunochromatographic assay for the qualitative detection of human chorionic gonadotropin (HCG) levels in human serum and urine, it is used for early pregnancy diagnosis This test is a screening reagent. All positive sample must be confirmed by other methodologies. This test is intended for healthcare professional use only.
PACKAGE SIZE
1 kit /box, 10 kits /box, 25 kits,/box, 50 kits /box.
SUMMARY
HCG is a glycoprotein hormone secreted by the developing placenta after egg fertilization. HCG levels can be rapidly elevated in serum or urine as early as 1 to 2.5 weeks during pregnancy, and reach a peak in 8 week, than fall to medium level in 4 month, and maintained the level until the end of the pregnancy[1]. The Kit is a simple, visual qualitative test that detects HCG antigen in human serum or urine. The Diagnostic Kit is based on immunochromatography and can give a result within 15 minutes.
ASSAY PROCEDURE
1.Take out the test card from the foil bag, put it on the level table and mark it.
2.Discard the first two drops sample, add 3 drops (about 100μL) no bubble sample verticaly and slowly into sample well of the card with provided dispette, start timing.
3.The result should be read within 10-15 minutes, and it is invalid after 15 minutes.
Product detail pictures:

Related Product Guide:
Why Dutch hospitals are so good at beating superbugs | Calprotectin Elisa Kit
Evaluation of a New Immunochromatography Technology Test (LDBio Diagnostics) To Detect Toxoplasma IgG and IgM: Comparison with the Routine Architect Technique | Diagnostic Kit For Isoenzyme Mb Of C Reatine Kinase
We generally believe that one's character decides products' top quality, the details decides products' high-quality ,along with the REALISTIC,EFFICIENT AND INNOVATIVE team spirit for Manufactur standard Fob Psa Cea Afp Test Kit - Diagnostic Kit(Colloidal Gold)for Human Chorionic Gonadotrophin – Baysen , The product will supply to all over the world, such as: kazakhstan, Malta, Belgium, We confirm to public, cooperation, win-win situation as our principle, adhere to the philosophy of make a living by quality, keep developing by honesty , sincerely hope to build up a good relationship with more and more customers and friends, to achieve a win-win situation and common prosperity.
Good quality and fast delivery, it's very nice. Some products have a little bit problem, but the supplier replaced timely, overall, we are satisfied.