New Arrival China Cea Tumor Marker Test Kit - Diagnostic Kit (Colloidal Gold) for IgM/IgG Antibody to Dengue Virus – Baysen
New Arrival China Cea Tumor Marker Test Kit - Diagnostic Kit (Colloidal Gold) for IgM/IgG Antibody to Dengue Virus – Baysen Detail:
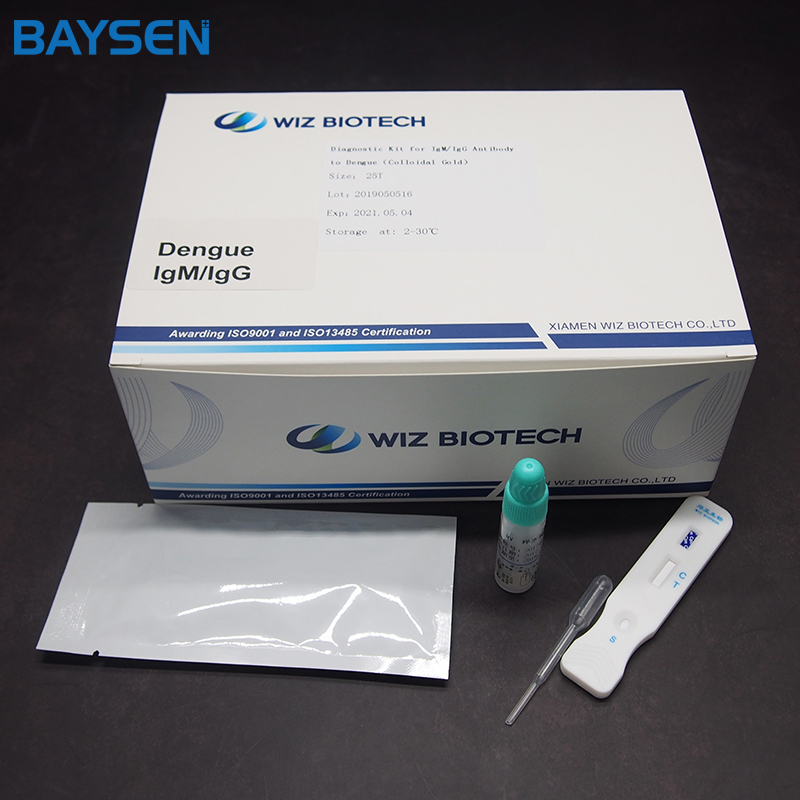
Diagnostic Kit (Colloidal Gold) for IgM/IgG Antibody to Dengue Virus
For in vitro diagnostic use only
Please read this package insert carefully prior to use and strictly follow the instructions. Reliability of assay results cannot be guaranteed if there are any deviations from the instructions in this package insert.
INTENDED USE
Diagnostic Kit(Colloidal Gold)for IgM/IgG Antibody to Dengue Virus is a colloidal gold immunochromatographic assay for the qualitative detection of IgM/IgG Antibody to Dengue Virus in human serum and plasma. This test is a screening reagent. All positive sample must be confirmed by other methodologies. This test is intended for healthcare professional use only.
PACKAGE SIZE
Twenty-five serving.
SUMMARY
Dengue Fever (Dengue Fever) is caused by Dengue virus infection. Dengue Fever is a group B vector virus and the yellow virus family flavivirus. Dengue virus antibody tests are often used enzyme-linked Immunoassay, Indirect Immunofluorescent Assay, Immunochromato – graphic Test. In recent years, with the development of the immune colloidal gold chromatography technology, using colloidal gold immune chromatography to detect serum (plasma) dengue virus antibody has become a reality. This method is characterized by simple operation, quick detection speed and saving manpower and material resources. It makes up for the inconveniences of ELISA and chemiluminescence method.
ASSAY PROCEDURE
1.Take out the test card from the foil bag, put it on the level table and mark it.
2.Use pipette to absorb sample 2ul, add it into sample well of the card;
3.Add 2 drops (80-100ul) sample diluent into sample well of the card;
4.The result should be read within 20 minutes.
TEST RESULTS AND INTERPRETATION[2]
Product detail pictures:


Related Product Guide:
Commutability of the First World Health Organization International Standard for Human Cytomegalovirus | Cpn-Igm
Who should get the PSA test for prostate cancer? | Diagnostic Kit For Isoenzyme Mb Of C Reatine Kinase
We attempt for excellence, provider the customers", hopes to be the most beneficial cooperation team and dominator enterprise for staff, suppliers and shoppers, realizes value share and continuous advertising for New Arrival China Cea Tumor Marker Test Kit - Diagnostic Kit (Colloidal Gold) for IgM/IgG Antibody to Dengue Virus – Baysen , The product will supply to all over the world, such as: Johannesburg, Hyderabad, Costa rica, With the goal of "zero defect". To care for the environment, and social returns, care employee social responsibility as own duty. We welcome friends from all over the world to visit and guide us so that we can achieve the win-win goal together.
The company can think what our think, the urgency of urgency to act in the interests of our position, can be said this is a responsible company, we had a happy cooperation!