Newly Arrival Malaria Pf/pv Antibody Test - PRINCIPLE AND PROCEDURE OF HbA1C TEST – Baysen
Newly Arrival Malaria Pf/pv Antibody Test - PRINCIPLE AND PROCEDURE OF HbA1C TEST – Baysen Detail:
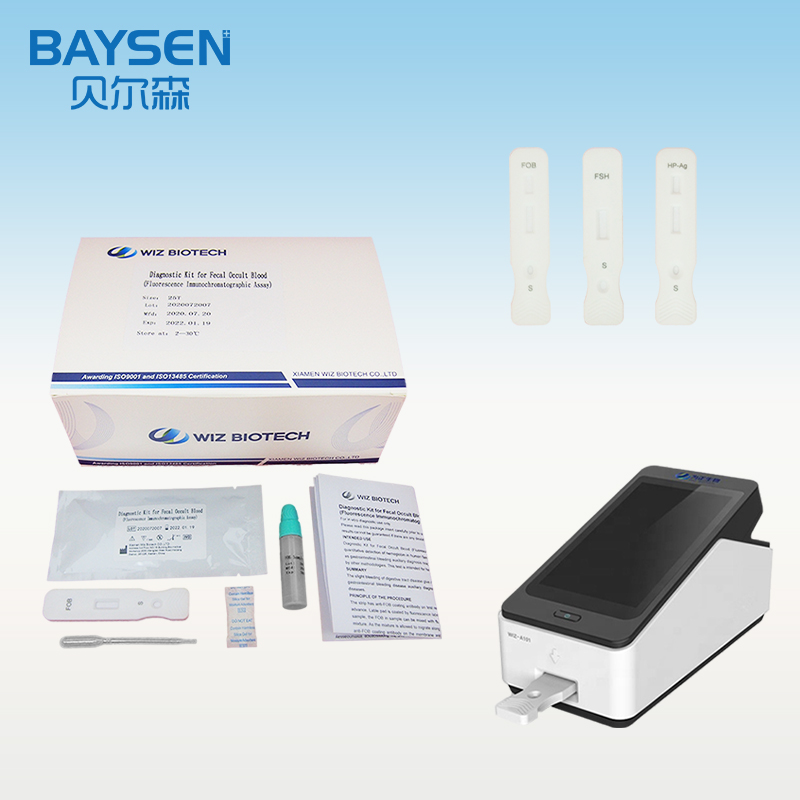
Products Parameters
PRINCIPLE AND PROCEDURE OF FOB TEST
PRINCIPLE
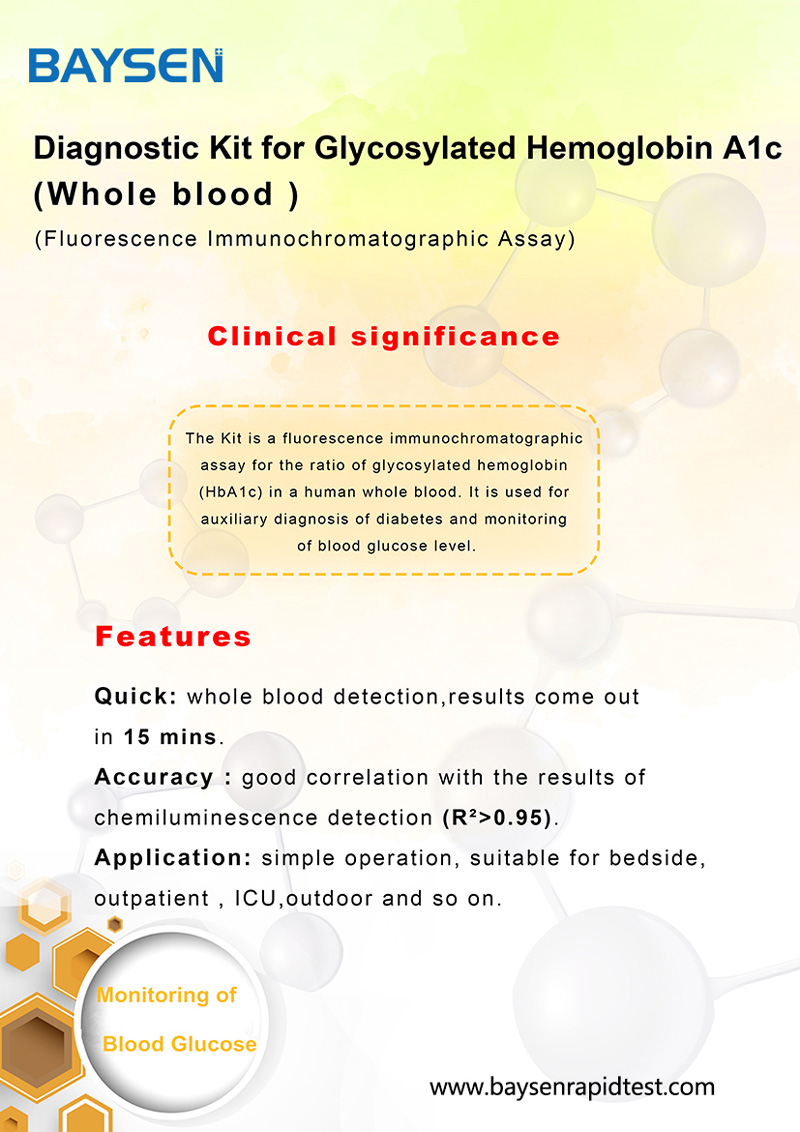
The membrane of the test device is coated with anti HbA1c antibody on the test region and anti Hb antibody on the control region. Lable pad are coated by fluorescence labeled anti Hb antibody and anti HbA1c antibody in advance. When testing sample, the HbA1c antigen in sample combine with fluorescence labeled anti HbA1c antibody, the Hb antigen in sample combine with fluorescence labeled anti Hb antibodyand form immune mixture. Under the action of the immunochromatography, the complex flow in the direction of absorbent paper, when complex passed the test region, it combined with anti HbA1c coating antibody, forms new complex. when complex passed the control region, it combined with anti Hb coating antibody, forms new complex.HbA1c level is positively correlated with fluorescence signal, and the concentration of HbA1c and Hb in sample can be detected by fluorescence immunoassay assay.
Test Procedure:
Please read the instrument operation manual and package insert before testing.
1. Lay aside all reagents and samples to room temperature.
2. Open the Portable Immune Analyzer(WIZ-A101), enter the account password login according to the operation method of the instrument, and enter the detection interface.
3. Scan the dentification code to confirm the test item.
4. Take out the test card from the foil bag.
5. Insert the test card into the card slot, scan the QR code, and determine the test item
6. Add 10μL Whole blood into sample diluent, shake 1 min and mix well.
7. Add 80μL sample solution to sample well of the card.
8. Click the “standard test” button, after 15 minutes, the instrument will automatically detect the test card, it can read the results from the display screen of the instrument, and record/print the test results.
9. Refer to the instruction of Portable Immune Analyzer(WIZ-A101).

About Us

Xiamen Baysen Medical Tech limited is a high biological enterprise which devotes itself to filed of fast diagnostic reagent and integrates research and development, production and sales into a whole. There are many advanced research staffs and sales managers in the company, all of them are have rich working experience in china and international biopharmaceutical enterprise.
Certificate display

Product detail pictures:



Related Product Guide:
Prostate cancer screening test costs Ontario patients $30 | Cpn-Igm
Cancer doctors give new PSA testing advice for some men | Cpn-Igm
we could supply good quality goods, aggressive cost and very best purchaser assistance. Our destination is "You come here with difficulty and we supply you with a smile to take away" for Newly Arrival Malaria Pf/pv Antibody Test - PRINCIPLE AND PROCEDURE OF HbA1C TEST – Baysen , The product will supply to all over the world, such as: Benin, Burundi, Lisbon, We have a excellent team supplying professional service, prompt reply, timely delivery, excellent quality and best price to our customers. Satisfaction and good credit to every customer is our priority. We are sincerely looking forward to cooperate with customers all over the world. We believe we can satisfy with you. We also warmly welcome customers to visit our company and purchase our products.
A nice supplier in this industry, after a detail and careful discussion, we reached a consensus agreement. Hope that we cooperate smoothly.