Non-Invasive Testing Breakthrough: Fecal Calprotectin “Crosses Boundaries” to Aid Early Diagnosis of Upper GI Inflammation in Children
In the field of diagnosing pediatric digestive system diseases, endoscopy has long been the “gold standard” for determining upper gastrointestinal inflammation. However, this invasive examination is not only accompanied by physical discomfort for children, especially young ones, but also often brings great psychological fear and difficulty in cooperating. This makes many parents hesitant during the initial diagnosis and may miss the opportunity for early intervention.Recently, a new clinical research and application practice has brought exciting news: fecal calprotectin (FCP), a mature non-invasive indicator for the assessment of lower gastrointestinal diseases, is showing great potential in the early diagnosis of upper gastrointestinal inflammation in children, achieving a wonderful “crossover” from the “lower intestine” to the “upper intestine”.
From the Dilemma of the “Gold Standard” to the Dawn of Non-Invasive Testing
Upper gastrointestinal inflammations such as gastritis and gastroduodenitis are not uncommon in children, and their causes include infection, drugs, and stress reactions. Traditionally, diagnosis requires visual observation and tissue biopsy through gastroscopy, which is a complicated and invasive process. Non-invasive and convenient detection methods have always been the common expectation of clinicians and families of children with the disease. Fecal calprotectin is a protein that reflects neutrophil aggregation. When the gastrointestinal mucosa is inflamed, its concentration will increase significantly. Over the years, it has been widely used in the activity assessment of inflammatory bowel disease (IBD) and the differential diagnosis of irritable bowel syndrome (IBS), mainly for colon inflammation.
Scientific research confirms a solid basis for “cross-border” applications
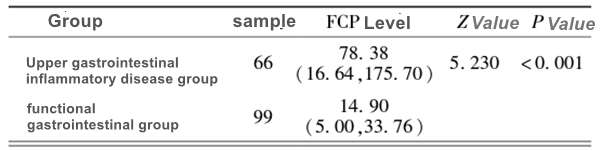
A growing body of research indicates that this inflammatory marker is not exclusive to the colon. When active inflammation occurs in the upper gastrointestinal tract (such as the stomach and duodenum), inflammatory cells also infiltrate and release calprotectin. This protein travels down the digestive tract along with digestive fluids and food residues, ultimately being detected in the stool. Recent studies in children have demonstrated that fecal calprotectin levels are significantly higher in children with endoscopically confirmed gastritis or duodenitis compared to those with functional dyspepsia or normal endoscopic findings.Although the elevated FC levels caused by upper gastrointestinal inflammation are generally lower than those in active IBD, they have shown statistically significant differences compared to healthy individuals. This suggests that FC testing can be used as an effective screening tool to help doctors initially identify children at high risk of organic upper gastrointestinal inflammation among a large number of children presenting with symptoms such as abdominal pain, bloating, and nausea.
Clinical Value: Building a Better Pediatric Diagnostic Pathway
The cross-border application of fecal calprotectin has brought multiple benefits to the diagnosis and management of upper gastrointestinal diseases in children:
1.Non-invasive and high compliance: Only a small amount of stool sample is required, and the process is completely non-invasive, which greatly reduces the physical and mental burden on children. Parents are highly accepting of the procedure, making it easy to quickly conduct repeated monitoring in outpatient clinics.
2. Effective screening and triage tool: For children with persistent gastrointestinal symptoms, Fecal Calprotectin testing can be performed first to effectively differentiate between inflammatory and functional diseases. If Fecal calprotectin levels are normal, functional factors can be prioritized or empirical treatment and observation can be adopted. If FC levels are elevated, it provides a strong basis for invasive gastroscopy, avoiding unnecessary endoscopic procedures and optimizing the allocation of medical resources.
3. Assisted evaluation of efficacy and recurrence: After the diagnosis of upper gastrointestinal inflammation and the start of treatment, dynamic monitoring of changes in Fecal Calprotectin levels can serve as an objective reference indicator for evaluating whether the inflammation has subsided and whether the treatment is effective. It can also help to detect disease recurrence early.
Future Outlook
Of course, the application of fecal calprotectin in the upper gastrointestinal tract still requires further research to precisely define its optimal cutoff value and rule out other lower gastrointestinal factors that may cause elevated FC. However, as a safe, simple, and low-cost screening method, it undoubtedly opens a new door for the early diagnosis of upper gastrointestinal inflammation in children. It marks a step forward in the diagnosis of pediatric digestive diseases towards a more humane and precise approach. We believe that with continued in-depth research and accumulated clinical experience, fecal calprotectin, this “crossover star,” will play an increasingly important role in protecting children’s digestive health.
Baysen Medical is always focus on diagnostic technique to improve the quality of life . We have developed 5 technology platforms- Latex , colloidal gold , Fluorescence Immunochromatographic Assay , Molecular,Chemiluminescence Immunoassay.We have Colloidal Gold Fecal calprotectin test kit and Fluorescence Immunoassay Calprotectin test kit for testing
Post time: Sep-23-2025