OEM China Universal Test Strip - Diagnostic Kit(LATEX)for Rotavirus Group A – Baysen
OEM China Universal Test Strip - Diagnostic Kit(LATEX)for Rotavirus Group A – Baysen Detail:
Diagnostic Kit(LATEX)for Rotavirus Group A
For in vitro diagnostic use only
Please read this package insert carefully prior to use and strictly follow the instructions. Reliability of assay results cannot be guaranteed if there are any deviations from the instructions in this package insert.
INTENDED USE
Diagnostic Kit(LATEX)for Rotavirus Group A is suitable for qualitative detection of Rotavirus Group A antigen in human fecal samples. This test is intended for healthcare professional use only. Meanwhile, this test is used for the clinical diagnosis of infantile diarrhea in patients with Rotavirus Group A infection.
PACKAGE SIZE
1 kit /box, 10 kits /box, 25 kits,/box, 50 kits /box.
SUMMARY
Rotavirus is classified as a rotavirus genus of the exenteral virus, which has a spherical shape with a diameter of about 70nm. Rotavirus contains 11 segments of double-stranded RNA. The rotavirus can be seven groups (a-g) based on antigenic differences and gene characteristics. Human infections of group A, group B and C group rotavirus have been reported. Rotavirus Group A is the important cause of severe gastroenteritis in children worldwide[1-2].
ASSAY PROCEDURE
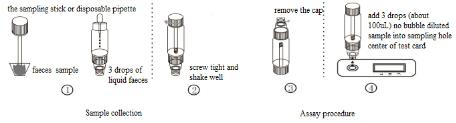
1.Take out the sampling stick, inserted into the faeces sample, then put the sampling stick back, screw tight and shake well, repeat the action 3 times. Or using the sampling stick picked about 50mg faeces sample, and put in a faeces sample tube containing sample dilution, and screw tightly.
2.Use disposable pipette sampling take the thinner faeces sample from the diarrhea patient, then add 3 drops (about 100uL) to the fecal sampling tube and shake well, put aside.
3.Take out the test card from the foil bag, put it on the level table and mark it.
4.Remove the cap from the sample tube and discard the first two drops diluted sample, add 3 drops (about 100uL) no bubble diluted sample verticaly and slowly into sample well of the card with provided dispette, start timing.
5.The result should be read within 10-15 minutes, and it is invalid after 15 minutes.
Product detail pictures:

Related Product Guide:
Guys: You Don’t Want That PSA Test For Prostate Cancer | Psa Test Cost
Benefits of PSA test to screen for prostate cancer are roughly equal to its harms, expert panel says | P24 Test Strips
The company keeps to the operation concept "scientific management, high quality and efficiency primacy, customer supreme for OEM China Universal Test Strip - Diagnostic Kit(LATEX)for Rotavirus Group A – Baysen , The product will supply to all over the world, such as: Portland, Peru, Macedonia, Now, with the development of internet, and the trend of internationalization, we have decided to extend business to overseas market. With the propose of bringing more profits to oversea customers by providing directly abroad. So we have changed our mind, from home to abroad, hope to give our customers more profit, and looking forward to more chance to make business.
The company comply with the contract strict, a very reputable manufacturers, worthy a long-term cooperation.