Reasonable price for Icare Chlamydia Rapid Screen Test - Infectious HIV HCV HBSAG AND Syphilish Rapid Combo Test – Baysen
Reasonable price for Icare Chlamydia Rapid Screen Test - Infectious HIV HCV HBSAG AND Syphilish Rapid Combo Test – Baysen Detail:
PRODUCTION INFORMATION
| Model Number | HBsAg/TP&HIV/HCV | Packing | 20 Tests/ kit, 30kits/CTN |
| Name | HBsAg/TP&HIV/HCV Rapid Combo Test | Instrument classification | Class III |
| Features | High sensitivity, Easy opeation | Certificate | CE/ ISO13485 |
| Accuracy | > 97% | Shelf life | Two Years |
| Methodology | Colloidal Gold | OEM/ODM service | Avaliable |
INTENDED USE
This kit is suitable for the in vitro qualitative determination of hepatitis B virus, syphilis spirochete, human immunodeficiency virus, and hepatitis C virus in human serum/plas- ma/whole blood samples for the auxiliary diagnosis of hepatitis B virus, syphilis spirochete, human immunodeficiency virus, and hepatitis C virus infections. The results obtained should be analyzed in conjunction with other clinical information. It is intended for use by medical professionals only.
Test procedure
| 1 | Read the instruction for use and in strict conformity with instruction for use required operation to avoid affecting the accuracy of the test results |
| 2 | Before the test, the kit and the sample are taken out from thestorage condition and balanced to room temperature and mark it. |
| 3 | Tearing the packaging of the aluminum foil pouch, take out the test device and mark it, then place it horizontally on the test table. |
| 4 | Aspirate serum/plasma samples with a disposable dropper and add 2 drops in each of wells s1 and s2; add 3 drops in each of wells s1 and s2 for whole blood samples before adding 1~2 drops of rinse solution to each of wells s1 and s2 and the Timing is started |
| 5 | Test results should be interpreted within 15~20 minutes, if more than 20 min interpreted results are invalid. |
| 6 | Visual interpretation can be used in result interpretation. |
Note: each sample shall be pipetted by clean disposable pipette to avoid cross contamination.
CLINICAL PERFORMANCE
| WIZ Results ofHBsag | Test result of Reference reagent | Positive coincidence rate:99.06% (95%C.I. 96.64%~99.74%) Negative coincidence rate:98.69% (95%C.I.96.68%~99.49%) Total coincidence rate:98.84% (95%C.I.97.50%~99.47% |
||
| Positive | Negative | Total | ||
| Positve | 211 | 4 | 215 | |
| Negative | 2 | 301 | 303 | |
| Total | 213 | 305 | 518 | |
| WIZ Results ofTP | Test result of Reference reagent | Positive coincidence rate:96.18% (95%C.I. 91.38%~98.36%) Negative coincidence rate:97.67% (95%C.I.95.64%~98.77%) Total coincidence rate:97.30% (95%C.I.95.51%~98.38%) |
||
| Positive | Negative | Total | ||
| Positve | 126 | 9 | 135 | |
| Negative | 5 | 378 | 383 | |
| Total | 131 | 387 | 518 | |
| WIZ Results ofHCV | Test result of Reference reagent | Positive coincidence rate:93.44% (95%C.I. 84.32%~97.42%) Negative coincidence rate:99.56% (95%C.I.98.42%~99.88%) Total coincidence rate:98.84% (95%C.I.97.50%~99.47%) |
||
| Positive | Negative | Total | ||
| Positve | 57 | 2 | 59 | |
| Negative | 4 | 455 | 459 | |
| Total | 61 | 457 | 518 | |
| WIZ Results ofHIV | Test result of Reference reagent | Positive coincidence rate:96.81% (95%C.I. 91.03%~98.91%) Negative coincidence rate:99.76% (95%C.I.98.68%~99.96%) Total coincidence rate:99.23% (95%C.I.98.03%~99.70%) |
||
| Positive | Negative | Total | ||
| Positve | 91 | 1 | 92 | |
| Negative | 3 | 423 | 446 | |
| Total | 94 | 424 | 518 | |
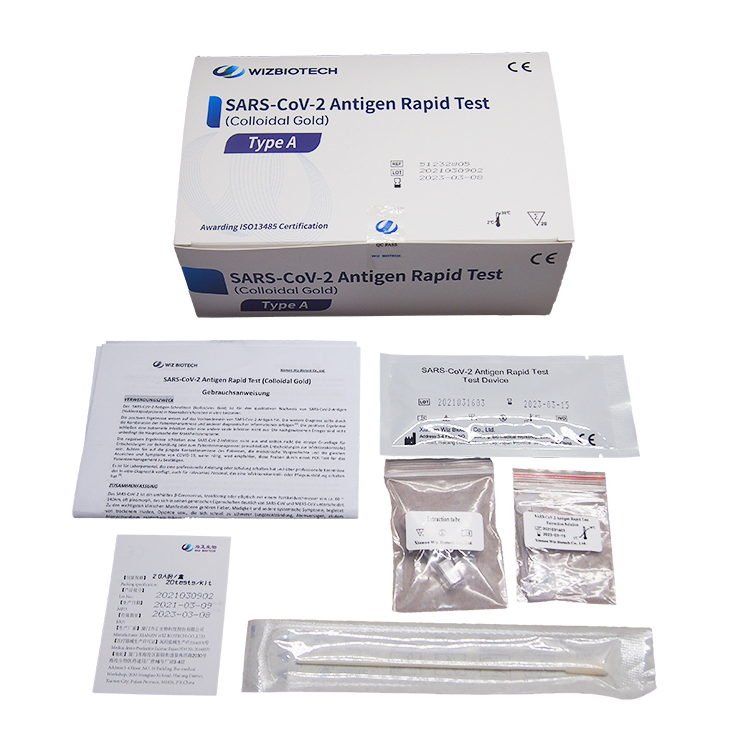
Product detail pictures:

Related Product Guide:
Experts on what needs to be done on prostate cancer | Diagnostic Kit For Isoenzyme Mb Of C Reatine Kinase
Most Men Don’t Need a PSA Test for Prostate Cancer | Cpn-Igm
Sticking for the basic principle of "Super Top quality, Satisfactory service" ,We've been striving to be an excellent business enterprise partner of you for Reasonable price for Icare Chlamydia Rapid Screen Test - Infectious HIV HCV HBSAG AND Syphilish Rapid Combo Test – Baysen , The product will supply to all over the world, such as: Mozambique, Casablanca, Bolivia, We welcome an opportunity to do business with you and hope to have pleasure in attaching further details of our products. Excellent quality, competitive price, punctual delivery and dependable service can be guaranteed. For further inquires please do not hesitate to contact us.
The customer service staff's attitude is very sincere and the reply is timely and very detailed, this is very helpful for our deal,thank you.