Renewable Design for CE approved monkeypox test - Diagnostic Kit for Cardiac Troponin I Myoglobin and Isoenzyme MB of Creatine Kinase – Baysen
Renewable Design for CE approved monkeypox test - Diagnostic Kit for Cardiac Troponin I Myoglobin and Isoenzyme MB of Creatine Kinase – Baysen Detail:
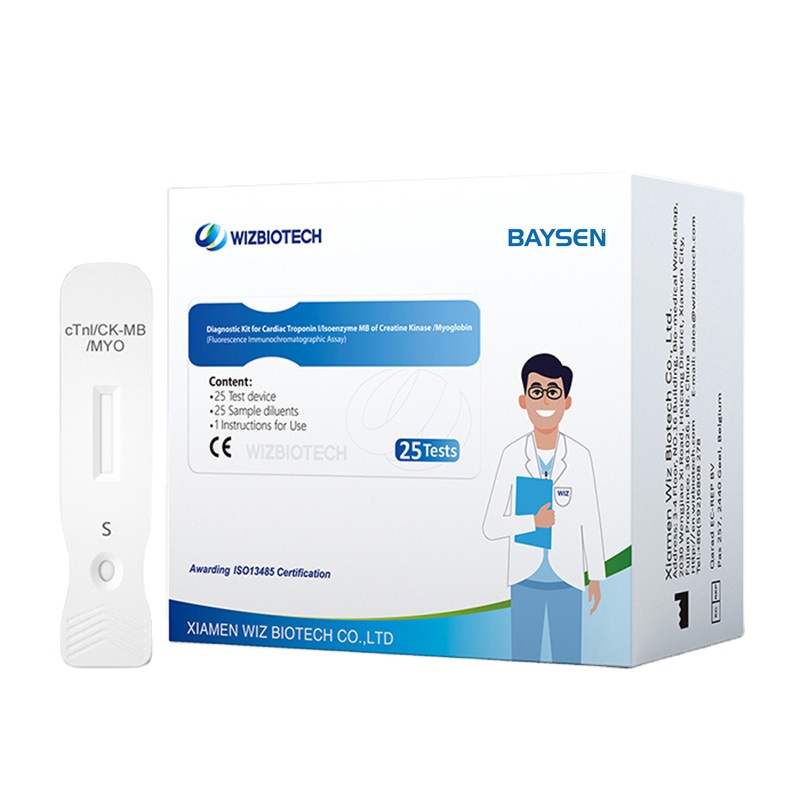
Diagnostic Kit for Cardiac Troponin I ∕Isoenzyme MB of Creatine Kinase ∕Myoglobin
Methodology:Fluorescence Immunochromatographic Assay
Production information
| Model Number | cTnI/CK-MB/MYO | Packing | 25 Tests/ kit, 30kits/CTN |
| Name | Diagnostic Kit for Cardiac Troponin I ∕Isoenzyme MB of Creatine Kinase ∕Myoglobin | Instrument classification | Class II |
| Features | High sensitivity, Easy opeation | Certificate | CE/ ISO13485 |
| Accuracy | > 99% | Shelf life | Two Years |
| Methodology | Fluorescence Immunochromatographic Assay | OEM/ODM service | Avaliable |
INTENDED USE
This kit is applicable to in vitro quantitative detection of concentrations of myocardial injury markers of cardiac
troponin I, isoenzyme MB of creatine kinasein and myoglobin in human serum/plasma/whole blood sample, and
it’s suitable for auxiliary diagnosis of myocardial infarction. This kit only provides test results of cardiac troponin I,
isoenzyme MB of creatine kinasein and myoglobin, and results obtained shall be used in combination with other
clinical information for analysis. It must only be used by healthcare professionals.
Test procedure
| 1 | Before using the reagent,read the package insert carefully and familiarize yourself with the operating procedures. |
| 2 | Select standard test mode of WIZ-A101 portable immune analyzer |
| 3 | Open the aluminum foil bag package of reagent and take out the test device. |
| 4 | Horizontally insert the test device into the slot of immune analyzer. |
| 5 | On home page of operation interface of immune analyzer, click “Standard” to enter test interface. |
| 6 | Click “QC Scan” to scan the QR code on inner side of the kit; input kit related parameters into instrument and select sample type. Note: Each batch number of the kit shall be scanned for one time. If the batch number has been scanned, then skip this step. |
| 7 | Check the consistency of “Product Name”, “Batch Number” etc. on test interface with information on the kit label. |
| 8 | Take out sample diluent upon consistent information, add 80μL serum/plasma/whole blood sample, and thoroughly mix them; |
| 9 | Add 80µL aforesaid thoroughly mixed solution into well of test device; |
| 10 | After complete sample addition, click “Timing” and remaining test time will be automatically displayed on the interface. |
| 11 | Immune analyzer will automatically complete test and analysis when test time is reached. |
| 12 | After test by immune analyzer is completed, test result will be displayed on test interface or can be viewedthrough “History” on home page of operation interface. |
Note: each sample shall be pipetted by clean disposable pipette to avoid cross contamination.
Product detail pictures:


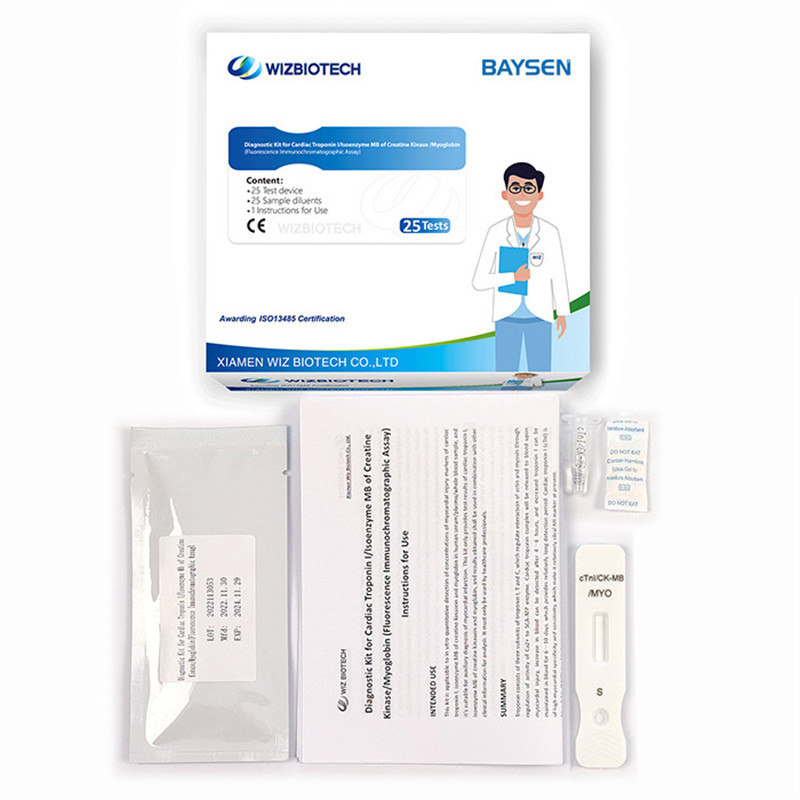
Related Product Guide:
Myeloid-related protein 8 induces self-tolerance and cross-tolerance to bacterial infection via TLR4- and TLR2-mediated signal pathways | Calprotectin Elisa Kit
MDxHealth (R): SelectMDx Cost-effective in Four European Countries Brussels Stock Exchange:MDXH | Calprotectin Elisa Kit
Well-run equipment, expert income workforce, and far better after-sales expert services; We are also a unified large family, anyone stick to the corporate value "unification, dedication, tolerance" for Renewable Design for CE approved monkeypox test - Diagnostic Kit for Cardiac Troponin I Myoglobin and Isoenzyme MB of Creatine Kinase – Baysen , The product will supply to all over the world, such as: UK, Kyrgyzstan, Mauritania, We follow up the career and aspiration of our elder generation, and we are eager to open up a new prospect in this field, We insist on "Integrity, Profession, Win-win Cooperation", because we have a strong backup, that are excellent partners with advanced manufacturing lines, abundant technical strength, standard inspection system and good production capacity.
The sales manager has a good English level and skilled professional knowledge, we have a good communication. He is a warm and cheerful man, we have a pleasant cooperation and we became very good friends in private.