Renewable Design for CE approved monkeypox test - quantitative kit CEA rapid test kit made in china factory supply – Baysen
Renewable Design for CE approved monkeypox test - quantitative kit CEA rapid test kit made in china factory supply – Baysen Detail:
Products Parameters

PRINCIPLE AND PROCEDURE OF FOB TEST
PRINCIPLE
The membrane of the test device is coated with anti CEA antibody on the test region and goat anti rabbit IgG antibody on the control region. Lable pad are coated by fluorescence labeled anti CEA antibody and rabbit IgG in advance. When testing positive sample, the CEA antigen in sample combine with fluorescence labeled anti CEA antibody, and form immune mixture. Under the action of the immunochromatography, the complex flow in the direction of absorbent paper, when complex passed the test region, it combined with anti CEA coating antibody, forms new complex.CEA level is positively correlated with fluorescence signal, and the concentration of CEA in sample can be detected by fluorescence immunoassay assay.
Test Procedure:
Please read the instrument operation manual and package insert before testing.
1. Lay aside all reagents and samples to room temperature.
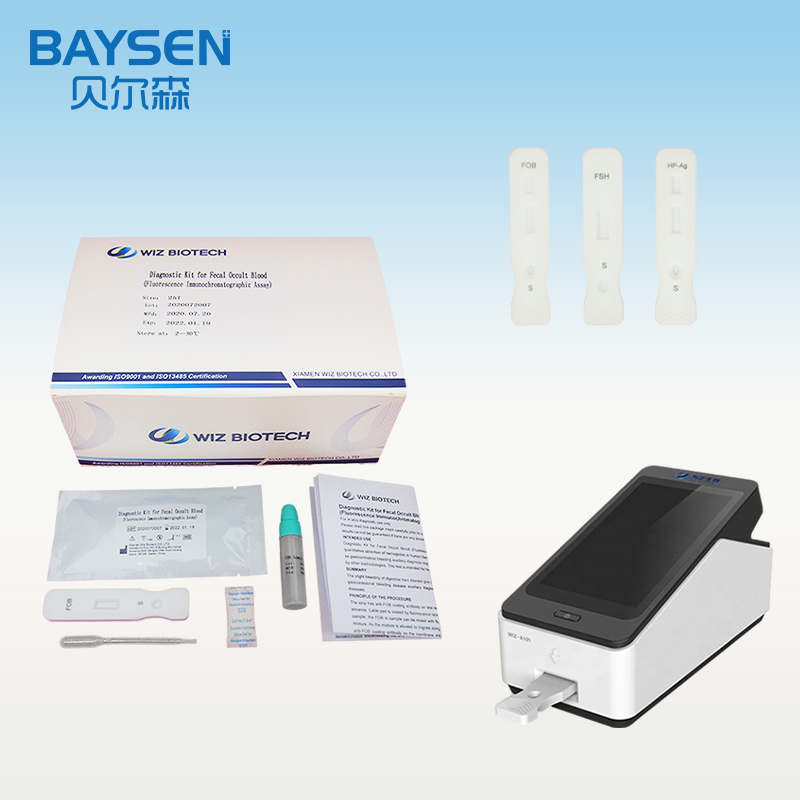
2. Open the Portable Immune Analyzer(WIZ-A101), enter the account password login according to the operation method of the instrument, and enter the detection interface.
3. Scan the dentification code to confirm the test item.
4. Take out the test card from the foil bag.
5. Insert the test card into the card slot, scan the QR code, and determine the test item.
6. Add 80μL serum or plasma sample into sample diluent, and mix well.
7. Add 80μL sample solution to sample well of the card.
8. Click the “standard test” button, after 15 minutes, the instrument will automatically detect the test card, it can read the results from the display screen of the instrument, and record/print the test results.
9. Refer to the instruction of Portable Immune Analyzer(WIZ-A101).

You May like
SARS-CoV-2 Antigen Rapid Test(Colloidal Gold)
WIZ-A101 Portable Immune Analyzer
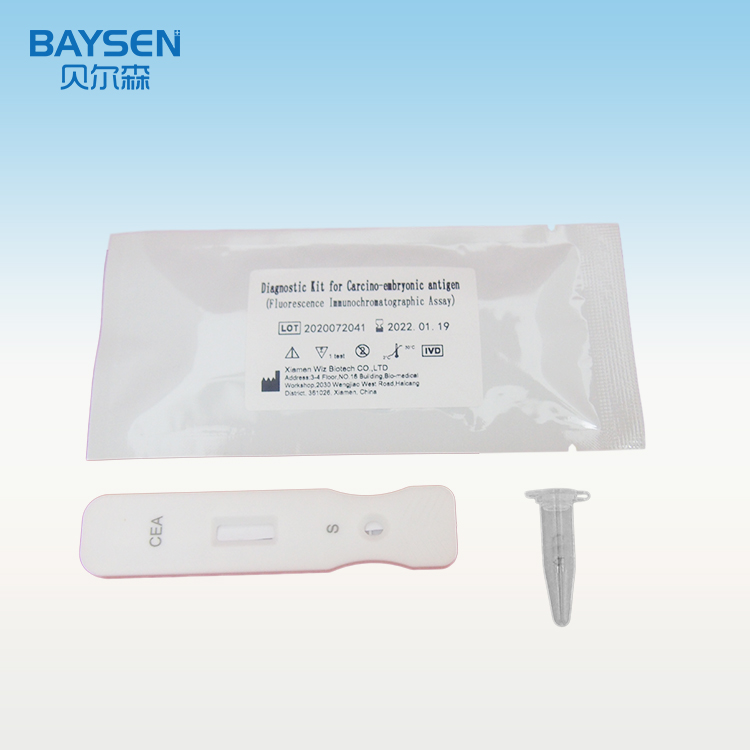
Diagnostic Kit for Carcomp-embrupmoc antigen (Fluorescence Immunochromatographic Assay)
About Us

Xiamen Baysen Medical Tech limited is a high biological enterprise which devotes itself to filed of fast diagnostic reagent and integrates research and development, production and sales into a whole. There are many advanced research staffs and sales managers in the company, all of them are have rich working experience in china and international biopharmaceutical enterprise.
Certificate display

Product detail pictures:


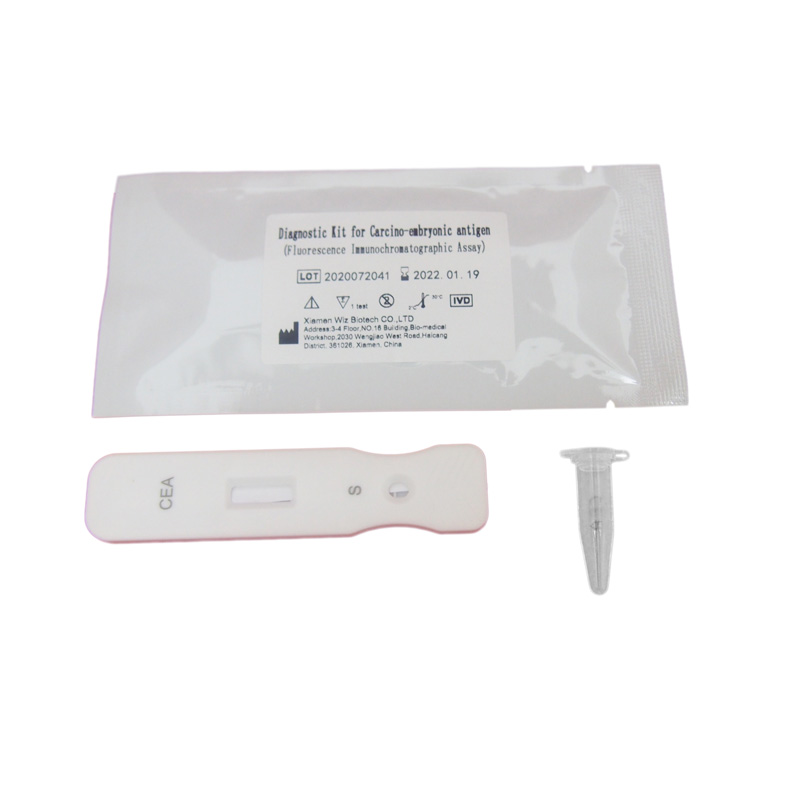

Related Product Guide:
Tests Show Promise for Informing Decisions About Prostate Cancer | P24 Test Strips
Even casual readers expect more than broad claims about screening tests. Where’s the data? | Cpn-Igm
To continually enhance the management technique by virtue of your rule of "sincerely, great faith and high-quality are the base of company development", we widely absorb the essence of similar merchandise internationally, and continuously build new merchandise to meet the demands of customers for Renewable Design for CE approved monkeypox test - quantitative kit CEA rapid test kit made in china factory supply – Baysen , The product will supply to all over the world, such as: Singapore, Iceland, Portland, To let customers be more confident in us and get the most comfortable service, we run our company with honesty, sincerity and best quality . We firmly believe that it is our pleasure to help customers to run their business more successfully, and that our professional advice and service can lead to more suitable choice for the customers.
It is a very good, very rare business partners, looking forward to the next more perfect cooperation!