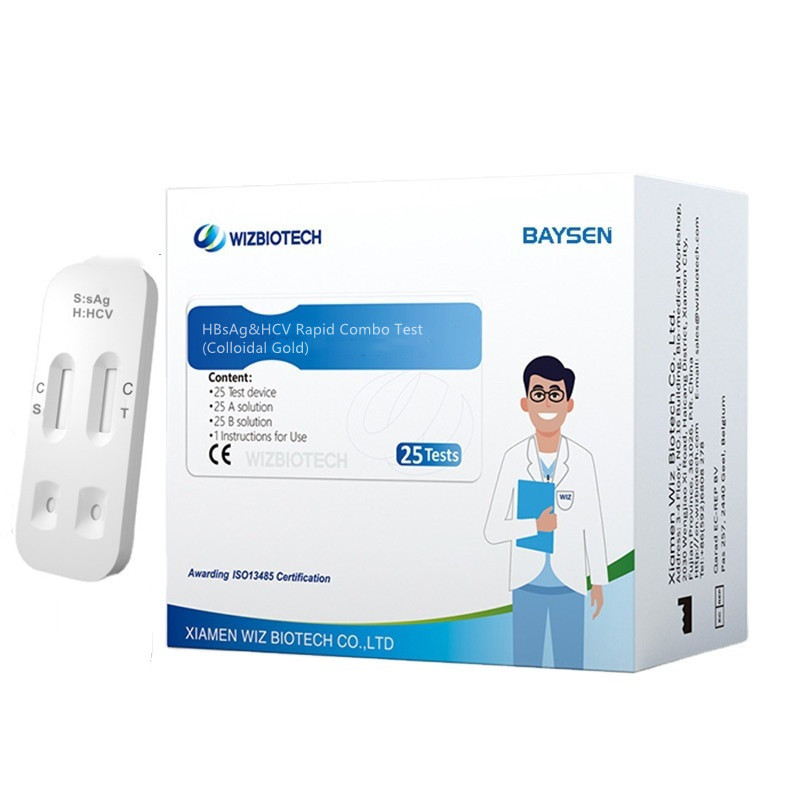
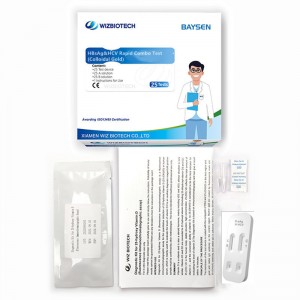
Colloidal Gold Blood HBsAg&HCV Rapid Combo Rapid Test
PRODUCTION INFORMATION
| Model Number | HBsAg&HCV Combo test | Packing | 20 Tests/ kit, 30kits/CTN |
| Name | HBsAg &HCV Rapid Combo Test |
Instrument classification | Class III |
| Features | High sensitivity, Easy opeation | Certificate | CE/ ISO13485 |
| Accuracy | > 97% | Shelf life | Two Years |
| Methodology | Colloidal Gold | OEM/ODM service | Avaliable |
Superiority
Testing time:15-20mins
Storage:2-30℃/36-86℉
Methodology:Colloidal Gold
Feature:
• High sensitive
• result reading in 15-20 minutes
• Easy operation
• High Accuracy
INTENDED USE
This kit is applicable to in vitro qualitative detection of hepatitis B virus and hepatitisC virus in human serum/plasma/whole blood sample, and it's suitable for auxiliary diagnosisof hepatitis B virus and hepatitis C virus infections, and is not suitable for blood screening. Theresults obtained should be analyzed in coniunction with other clinical information. itis intended for use by medical professionals only.
Test procedure
| 1 | Read the instruction for use and in strict conformity with instruction for use required operation to avoid affecting the accuracy of the test results |
| 2 | Before the test, the kit and the sample are taken out from thestorage condition and balanced to room temperature and mark it. |
| 3 | Tearing the packaging of the aluminum foil pouch, take out the test device and mark it, then place it horizontally on the test table. |
| 4 | The sample to be tested (serum/plasma) was added to S1 and S2 wells with 2 drops orthe sample to be tested (whole blood) was added to S1 and s2 wells with 3 drops. After thesample is added, 1 ~ 2 drops of sample dilution are added to the S1 and S2 wells and the timing is started. |
| 5 | Test results should be interpreted within 15~20 minutes, if more than 20 min interpreted results are invalid. |
| 6 | Visual interpretation can be used in result interpretation. |
Note: each sample shall be pipetted by clean disposable pipette to avoid cross contamination.
CLINICAL PERFORMANCE
| WIZ Results ofHBsag | Test result of Reference reagent | Positive coincidence rate :
99.48%(95%C.1.97.09%~99.91%) Negative coincidence rate : 99.25%(95%C.1.97.32%~99.80%) Total coincidence rate : 99.35%(95%C1.9810%~99.78%) |
||
| Positive | Negative | Total | ||
| Positve | 190 | 2 | 192 | |
| Negative | 1 | 266 | 267 | |
| Total | 191 | 268 | 459 | |
| WIZ Results ofHCV | Test result of Reference reagent |
Positive coincidence rate : 96.55%(95%C1.88.27%~99.05%) Negative coincidence rate : 99.50%(95%C.1.98.20%~99.86%) Total coincidence rate : 99.13%(95%C.1.97.78%~99.66%)
|
||
| Positive | Negative | Total | ||
| Positve | 56 | 2 | 58 | |
| Negative | 2 | 399 | 401 | |
| Total | 58 | 401 | 459 | |