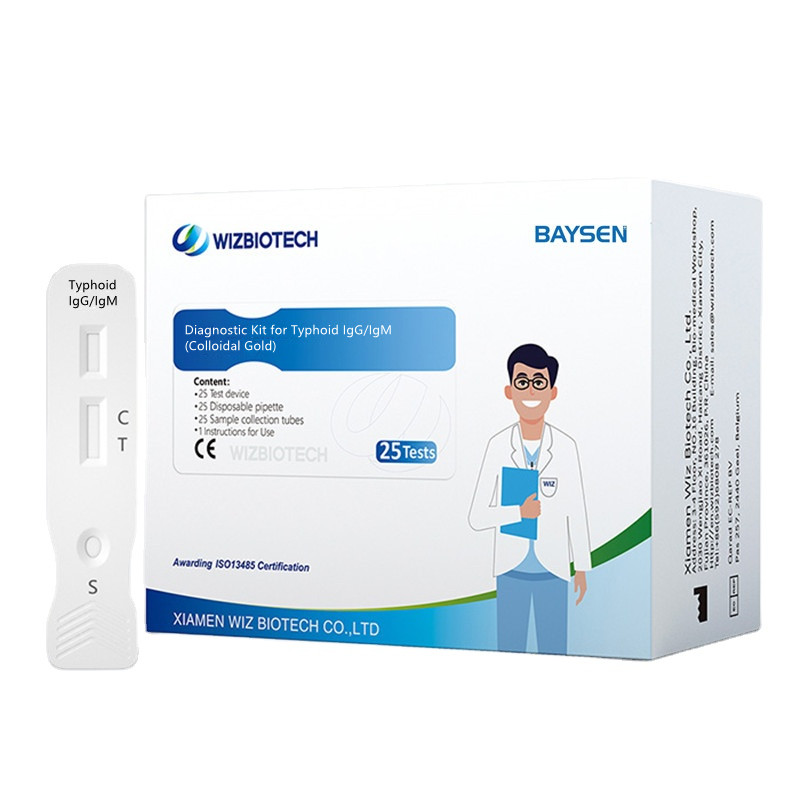
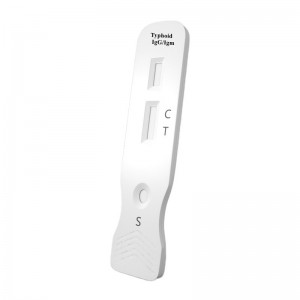
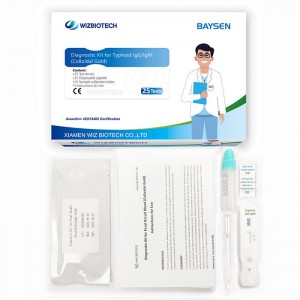
Colloidal Gold Blood Typhoid IgG/IgM Diagnostic Kit
Diagnostic Kit for Typhoid IgG/IgM
Colloidal Gold
Production information
| Model Number | Typhoid IgG/IgM | Packing | 25 Tests/ kit, 20kits/CTN |
| Name | Diagnostic Kit for Typhoid IgG/IgM | Instrument classification | Class Ii |
| Features | High sensitivity, Easy opeation | Certificate | CE/ ISO13485 |
| Accuracy | > 99% | Shelf life | Two Years |
| Methodology | Colloidal Gold | OEM/ODM service | Avaliable |
Test procedure
| 1 | Take out the test device from sealed foil pouch and place on a dry, clean and level surface |
| 2 | Be sure to label the device with specimen’s ID number |
| 3 | Fill the pipette dropper with the specimen. Hold the dropper vertically and transfer 1 drop of whole blood/serum/plasma specimen (approximately 10 μL) into the specimen well (S) , and make sure that there are no air bubbles. Then add 3 drops of sample diluent (approximately 80-100 μL) into the diluent well (D) immediately. See illustration below. |
| 4 |
Start the timer. |
| 5 | Wait for the colored line(s) to appear. Read test results at 15 minutes. Positive results maybe visible in as short as 1 minute. Negative results must be confirmed at the end of the 20 minutes only. Do not interpret the result after 20 minutes. |
Intend Use
Diagnostic Kit for Typhoid IgG/IgM (Colloidal Gold) is a rapid, serological, lateral flow chromatographic immunoassay designed for the simultaneous detection and differentiation of anti-Salmonella typhi (S.typhi) IgG and IgM in human whole blood, serum or plasma specimens. It is intended for use by healthcare professionals as a screening test and as an aid in diagnosing of infection with S. typhi. The test provides preliminary analysis results and do not serve as a definitive l diagnosis criterion. Any use or interpretation of the test must be analyzed and confirmed with alternative testing method(s) and clinical findings based on professional judgment of healthcare providers.
Superiority
Testing time:15 mins
Storage:2-30℃/36-86℉
Methodology:Colloidal Gold
CFDA Certificate
Feature:
• High sensitive
• result reading in 15 minutes
• Easy operation
• Factory direct price
• Do not need extra machine for result reading

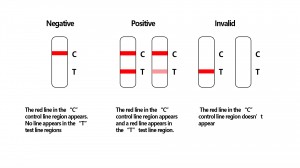
Result reading
The Typhoid IgG/IgM Rapid Test has been evaluated with a reference commercial ELISA test using clinical specimens. Test results are presented in the tables below:
Clinical performance for anti-S. typhi IgM Test
| WIZ result of Typhoid IgG/IgM | S. typhi IgM ELISA Test | Sensitivity (Positive Percent Agreement):
93.93% = 31/33 (95% CI: 80.39%~98.32%) Specificity (Negative Percent Agreement): 99.52% = 209/210 (95% CI: 93.75%~99.92%) Accuracy (Overall Percent Agreement): 98.76% = (31+209)/243 (95% CI: 96.43%~99.58%) |
||
| Positive | Negative | Total | ||
| Positive | 31 | 1 | 32 | |
| Negative | 2 | 209 | 211 | |
| Total | 33 | 210 | 243 | |
Clinical performance for anti-S. typhi IgG Test
| WIZ result of Typhoid IgG/IgM | S. typhi IgG ELISA Test | Sensitivity (Positive Percent Agreement):
88.57% = 31/35 (95% CI: 74.05%~95.46%) Specificity (Negative Percent Agreement): 99.54% = 219/220 (95% CI: 97.47%~99.92%) Accuracy (Overall Percent Agreement): 98.03% = (31+219)/255 (95% CI: 95.49%~99.16%) |
||
| Positive | Negative | Total | ||
| Positive | 31 | 1 | 32 | |
| Negative | 4 | 219 | 223 | |
| Total | 35 | 220 | 255 | |
You may also like: