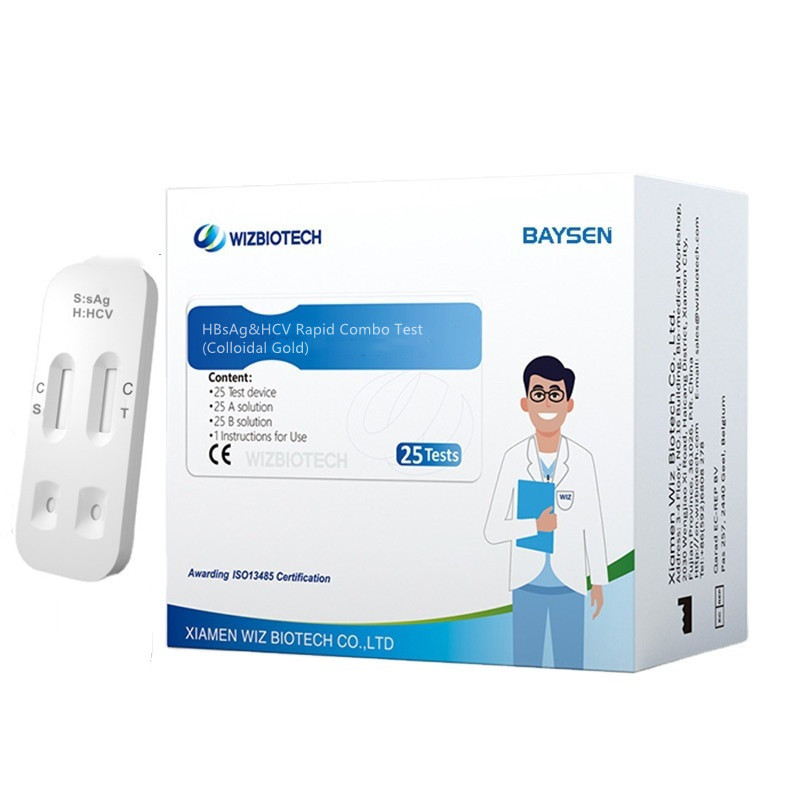
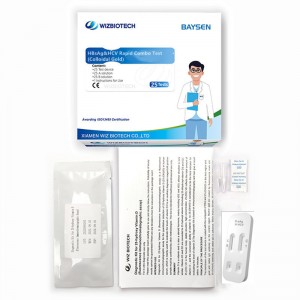
Colloidal Gold Blood HBsAg&HCV Gwajin Haɗaɗɗen Haɗaɗɗen Sauri
BAYANIN KYAUTA
| Lambar Samfura | Gwajin Combo HBsAg&HCV | Shiryawa | 20 Gwaje-gwaje/kit, 30kits/CTN |
| Suna | Gwajin Haɗaɗɗen Haɗaɗɗen HBsAg & HCV | Rarraba kayan aiki | Darasi na III |
| Siffofin | Babban hankali, Mai sauƙin aiki | Takaddun shaida | CE/ISO13485 |
| Daidaito | > 97% | Rayuwar rayuwa | Shekaru Biyu |
| Hanya | Colloidal Gold | OEM/ODM sabis | Akwai |
fifiko
Lokacin gwaji:15-20mins
Adana:2-30℃/36-86℉
Hanyar: Colloidal Gold
Siffa:
• Babban m
• karatun sakamako a cikin mintuna 15-20
• Sauƙi aiki
• Babban Daidaito
AMFANI DA NUFIN
Wannan kit ɗin yana aiki ne don gano in vitro qualitative detection of hepatitis B virus da hepatitisC virus a cikin jinin mutum/plasma/dukan samfurin jini, kuma ya dace da ƙarin bincike na cutar hanta B da ciwon hanta na C, kuma bai dace da gwajin jini ba. Ya kamata a yi nazarin sakamakon da aka samu tare da wasu bayanan asibiti. itis da aka yi nufin amfani da shi ta hanyar kwararrun likitoci kawai.
Hanyar gwaji
| 1 | Karanta umarnin don amfani kuma cikin cikakken tsari tare da umarni don amfani da aikin da ake buƙata don guje wa shafar daidaiton sakamakon gwajin. |
| 2 | Kafin gwajin, ana fitar da kit ɗin da samfurin daga yanayin ajiya kuma a daidaita su zuwa zafin daki kuma a yi masa alama. |
| 3 | Yaga marufi na jakar foil na aluminum, fitar da na'urar gwajin da alama, sannan a sanya shi a kwance akan teburin gwaji. |
| 4 | Samfurin da za a gwada (serum/plasma) an ƙara shi zuwa rijiyoyin S1 da S2 tare da ɗigo 2 ko samfurin da za a gwada (jini duka) an ƙara shi zuwa rijiyoyin S1 da s2 tare da digo 3. Bayan an ƙara samfurin, ana ƙara 1 ~ 2 digo na samfurin dilution a cikin rijiyoyin S1 da S2.an fara lokaci. |
| 5 | Ya kamata a fassara sakamakon gwajin a cikin mintuna 15 ~ 20, idan fiye da minti 20 sakamakon fassarar ba shi da inganci. |
| 6 | Ana iya amfani da fassarar gani a fassarar sakamako. |
Lura: kowane samfurin za a yi bututu ta hanyar pipette mai tsaftataccen zubarwa don guje wa gurɓacewar giciye.
AIYUKA na asibiti
| Sakamakon WIZHBsag | Sakamakon gwaji na Reagent Reagent | Mahimman ƙimar daidaituwa: 99.48% (95% C.1.97.09% ~ 99.91%) Adadin daidaituwa mara kyau: 99.25% (95% C.1.97.32% ~ 99.80%) Jimlar adadin daidaituwa: 99.35% (95% C1.9810% ~ 99.78%) | ||
| M | Korau | Jimlar | ||
| Tabbatacce | 190 | 2 | 192 | |
| Korau | 1 | 266 | 267 | |
| Jimlar | 191 | 268 | 459 | |
| Sakamakon WIZHCV | Sakamakon gwaji na Reagent Reagent | Mahimman ƙimar daidaituwa: 96.55% (95% C1.88.27% ~ 99.05%) Adadin daidaituwa mara kyau: 99.50% (95% C.1.98.20% ~ 99.86%) Jimlar adadin daidaituwa: 99.13% (95% C.1.97.78% ~ 99.66%)
| ||
| M | Korau | Jimlar | ||
| Tabbatacce | 56 | 2 | 58 | |
| Korau | 2 | 399 | 401 | |
| Jimlar | 58 | 401 | 459 | |