Diagnostic kit for Human Chorionic Gonadotropin pregnanacy test Colloidal Gold
Diagnostic Kit for Human Chorionic Gonadoteopin (Colloidal Gold)
Production information
| Model Number | HCG | Packing | 25 Tests/ kit, 30kits/CTN |
| Name | Diagnostic Kit for Human Chorionic Gonadoteopin (Colloidal Gold) | Instrument classification | Class I |
| Features | High sensitivity, Easy opeation | Certificate | CE/ ISO13485 |
| Accuracy | > 99% | Shelf life | Two Years |
| Methodology | Colloidal Gold | OEM/ODM service | Avaliable |
Test procedure
| 1 | Remove test device from aluminum foil pouch, lie it on a horizontal workbench, and do a good job in marking |
| 2 | Use disposable pipette to pipette serum/urine sample, discard first two drops of serum/urine, add 3 drops (approx. 100μL) of bubble-free serum/urine sample dropwise to well of test device vertically and slowly, and start counting time. |
| 3 | Interpret result within 10-15 minutes, and detection result is invalid after 15 minutes (see result in Diagram 2). |
Intend Use
This kit is applicable to in vitro qualitative detection of human chorionic gonadotropin (HCG) in serum sample, which’s suitable for auxiliary diagnosis of early trimester of pregnancy. This kit only provides human chorionic gonadotropin test results, and results obtained shall be used in combination with other clinical information for analysis. This kit is for healthcare professionals.

Summary
This kit is applicable to qualitative detection of human chorionic gonadotropin (HCG) in human urine and serum sample, which’s suitable for auxiliary diagnosis of early trimester of pregnancy. Mature women have embryo due to implantation of fertilized egg in uterine cavity, syncytiotrophoblast cells in placenta produce large amount of human chorionic gonadotrophin (HCG) during embryo's development into fetus, which can be excreted in the urine through blood circulation of pregnant women. HCG level in serum and urine can rise rapidly during 1~2.5 weeks of pregnancy, reach the peak at 8 weeks pregnant, reduce to intermediate level from 4 months pregnant, and maintain such level all the way to late pregnancy.
Feature:
• High sensitive
• result reading in 15 minutes
• Easy operation
• Factory direct price
• Do not need extra machine for result reading
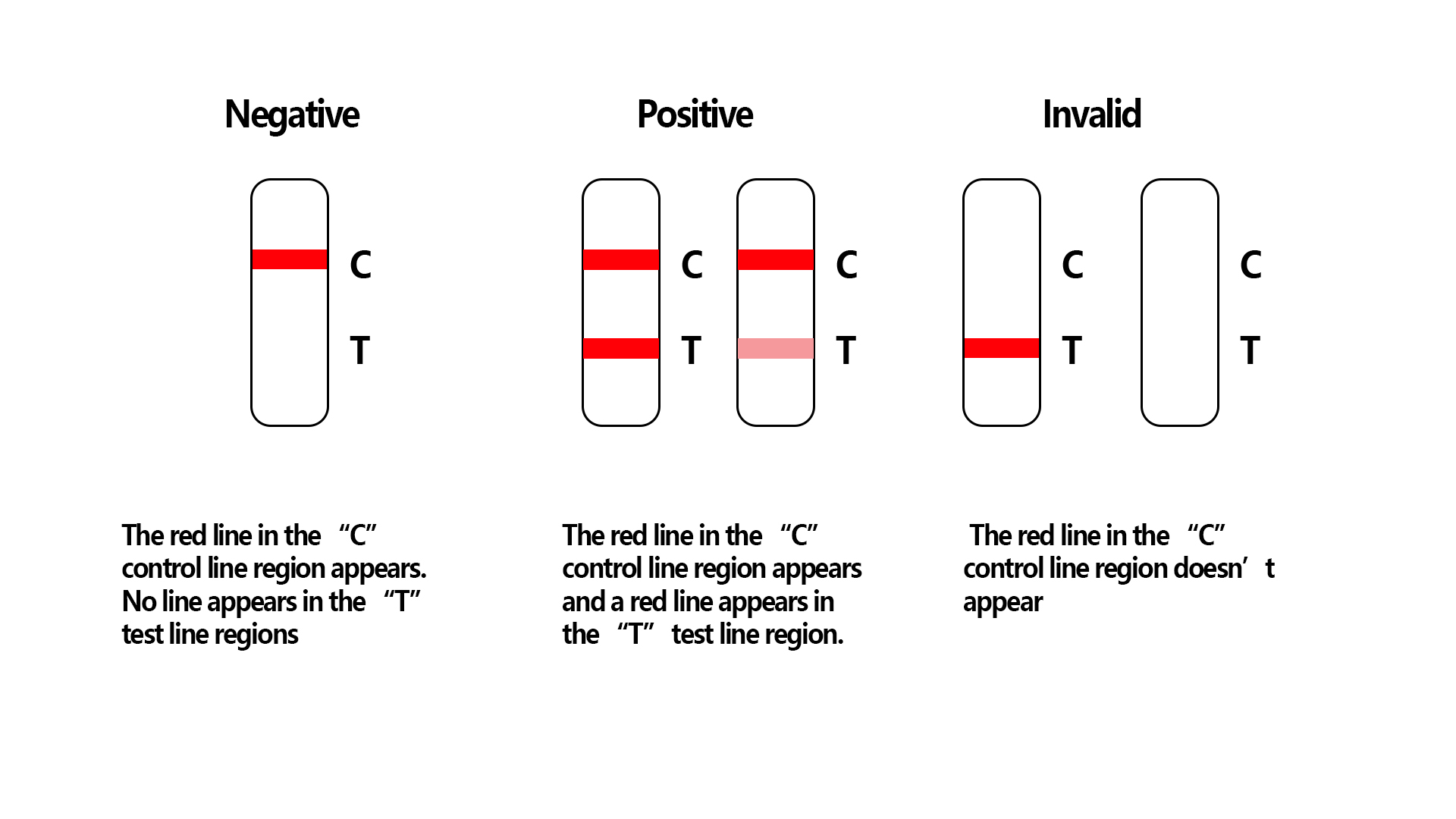
Result reading
The WIZ BIOTECH reagent test will be compared with the control reagent:
| WIZ results | Test resultof reference reagent | ||
| Positive | Negative | Total | |
| Positive | 166 | 0 | 166 |
| Negative | 1 | 144 | 145 |
| Total | 167 | 144 | 311 |
Positive coincidence rate:99.4%(95%C.I. 96.69%~99.89%)
Negative coincidence rate: 100%(95%C.I.97.40%~100%)
Total coincidence rate:99.68%(95%C.I.98.20%~99.40%)
You may also like:







-3.jpg)
-3-300x300.jpg)
-1-300x300.jpg)
-4-300x300.jpg)
-5-300x300.jpg)




-3-300x300.jpg)
-3-300x300.jpg)