Ngwa nyocha maka nje ịba ọcha n'anya C (Fluorescence Immunochromatographic Assay)
N'ihi na in vitro diagnostic ojiji naanị
Biko gụọ ihe ngwungwu a nke ọma tupu ejiri ya wee soro ntuziaka ahụ.Enweghị ike ịkwenye ntụkwasị obi nke nsonaazụ nyocha ma ọ bụrụ na ọ dị iche na ntuziaka dị na ntinye ngwugwu a.
Ezubere iji
Diagnostic Kit maka ịba ọcha n'anya C Virus Antibody (Fluorescence Immunochromatographic Assay) bụ a fluorescence immunochromatographic assay maka quantitative nchọpụta nke HCV antibody na mmadụ serum ma ọ bụ plasma, nke dị mkpa inyeaka diagnostic uru maka ọrịa na ịba ọcha n'anya C. All nti sample ga-ekwenyere site na ndị ọzọ. usoro.Ezubere ule a maka naanị ndị ọkachamara ahụike
1.Dịna n'akụkụ niile reagents na sample na ụlọ okpomọkụ.
2.Mepee Portable Immune Analyzer (WIZ-A101), tinye nbanye paswọọdụ akaụntụ dị ka usoro ọrụ nke ngwá ọrụ si dị, ma tinye interface nchọpụta ahụ.
3. Nyochaa koodu nyocha iji kwado ihe nlele ahụ.
4.Wepụ kaadị ule site na akpa foil.
5.Tinye kaadị ule n'ime oghere kaadị, nyochaa koodu QR, ma chọpụta ihe nlele ahụ.
6.Add 20μL serum ma ọ bụ plasma sample ka sample diluent, na mix ọma ..
7.Add 80μL sample ngwọta ka ịlele nke ọma nke kaadị.
8.Click bọtịnụ "ọkọlọtọ ule", mgbe nkeji iri na ise gachara, ngwa ahụ ga-achọpụta kaadị ule na-akpaghị aka, ọ nwere ike ịgụ ihe si na ihuenyo ngosi nke ngwá ọrụ ahụ, ma dekọọ / bipụta nsonaazụ ule.
9. Rụtụ aka na ntuziaka nke Portable Immune Analyzer(WIZ-A101).
Nchịkọta
Ọrịa ịba ọcha n'anya C (HCV) bụ envelopu, nje virus RNA (9.5kb) nke ezinaụlọ Flaviviridae nwere.Achọpụtala ụdị genotypes isii na usoro nke subụdị nke HCV.N'ịbụ nke dịpụrụ adịpụ na 1989, a ghọtara HCV ugbu a dị ka isi ihe kpatara mmịnye ọbara metụtara ịba ọcha n'anya na-abụghị A, na-abụghị B.A na-eji ụdị ọrịa ahụ mara nke ukwuu na nke na-adịghị ala ala.Ihe karịrị 50% nke ndị butere ọrịa ahụ na-etolite ịba ọcha n'anya na-adịghị ala ala nke nwere ọrịa imeju imeju na carcinoma hepatocellular.Kemgbe mmalite nke 1990 nke nyocha mgbochi HCV nke inye onyinye ọbara, ebelatala nke ukwuu nke ibute ọrịa a na ndị natara mmịnye ọbara.Nnyocha ụlọ ọgwụ na-egosi na ọnụ ọgụgụ dị ukwuu nke ndị butere ọrịa HCV na-emepụta ọgwụ mgbochi nje na protein NS5 na-abụghị nke nje.Maka nke a, ule ahụ gụnyere antigens sitere na mpaghara NS5 nke genome viral na mgbakwunye na NS3 (c200), NS4 (c200) na Core (c22).
Ụkpụrụ nke usoro
A na-ekpuchi akpụkpọ ahụ nke ngwaọrụ ule na HCV antigen na mpaghara ule na ewu mgbochi oke bekee IgG na mpaghara nchịkwa.A na-eji fluorescence nke akpọrọ HCV antigen na oke bekee IgG kpuchie akwa akwa akwa n'ihu.Mgbe a na-anwale nlele dị mma, ihe mgbochi HCV dị na sample na-ejikọta ya na fluorescence akpọrọ HCV antigen, wee mepụta ngwakọta mgbochi.N'okpuru omume nke immunochromatography, mgbagwoju eruba na ntụziaka nke absorbent akwụkwọ, mgbe mgbagwoju gafere ule mpaghara, ọ jikọtara ya na HCV antigen mkpuchi antigen, Nleta ọhụrụ complex.HCV antibody larịị na-kwekọrọ na fluorescence mgbaàmà, na ịta nke Enwere ike ịchọpụta ọgwụ mgbochi HCV na nlele site na fluorescence immunoassay assay
Reagent na ihe ndị e nyere
Ngwa ngwugwu 25T:
.Kaadị ule n'otu n'otu n'otu n'otu foil nke e ji ihe eji akwọcha akpa juru
.Sample diluents
.Ngwunye ntinye
AKWỤKWỌ KWESỊRỊ MA Ọ BỤGHỊ
Akpa mkpokọta ihe nlele, ngụ oge
NKWUKWU NA NKWUKWU
1.The samples nwalere nwere ike ịbụ serum, heparin anticoagulant plasma ma ọ bụ EDTA anticoagulant plasma.
2.According ọkọlọtọ usoro na-anakọta sample.Enwere ike idowe ọbara ọbara ma ọ bụ plasma refrigerated na 2-8 ℃ maka 7days na cryopreservation n'okpuru -15 Celsius C maka ọnwa 6.
3.All sample zere ifriizi-thaw cycles.
Usoro ASsay
Biko gụọ akwụkwọ ntuziaka ọrụ akụrụngwa na ntinye ngwugwu tupu ịnwale.
.Nnwale ule a bụ naanị maka ntụaka ụlọ ọgwụ, ekwesịghị ịbụ naanị ihe ndabere maka nyocha na ọgwụgwọ ụlọ ọgwụ, ndị na-ahụ maka ụlọ ọgwụ na-ahụ maka ndị ọrịa kwesịrị ịdị na-atụgharị uche zuru oke na-ejikọta ya na mgbaàmà ya, akụkọ ahụike ahụike, nyocha ụlọ nyocha ndị ọzọ, nzaghachi ọgwụgwọ, ọrịa na-efe efe na ozi ndị ọzọ. .
A na-eji reagent naanị maka nyocha ọbara na plasma.O nwere ike ọ gaghị enweta nsonaazụ ziri ezi mgbe ejiri ya mee ihe maka nlele ndị ọzọ dị ka mmiri mmiri na mmamịrị na wdg.
ÀJỤMỤ ỌRỤ
| Linearity | 0.005-5 | Ngbanwe nke ikwu: -15% ruo +15%. |
| Ọnụọgụ njikọ linear:(r)≥0.9900 | ||
| Izi ezi | Ọnụego mgbake ga-adị n'ime 85% - 115%. | |
| Nkwagharị ugboro ugboro | CV≤15% | |
Ntụaka
1.Mịnye ọbara ịba ọcha n'anya.Na: Moore SB, ed.Ọrịa nje na-ebufe mmịnye ọbara.Alington, VA.M.Assoc.Ụlọ akụ ọbara, p. 53-38.
2.Hansen JH, et al.HAMA Ntinye aka na Murine Monoclonal Antibody Based Immunoassays[J].J nke Clin Immunoassay,1993,16:294-299.
3.Levinson SS. Ọdịdị nke Antibodies Heterophilic na Ọrụ dị na Ntinye aka Immunoassay [J].J nke Clin Immunoassay,1992,15:108-114.
4.Alter HJ., Purcell RH, Holland PV, et al.(1978) Ndị nnọchi anya na-ebugharị na ịba ọcha n'anya na-abụghị A, na-abụghị B.Lancet I: 459-463.
5.Choo QL,Weiner AJ, Overby LR, Kuo G, Houghton M. (1990) Nje Virus ịba ọcha n'anya C: isi ihe na-akpata nje na-abụghị A, ịba ọcha n'anya na-abụghị B.Br Med Bull 46: 423-441 .
6.Engvall E, Perlmann P. (1971) Enzyme jikọtara immunosorbent assay (ELISA): qualitative assay of IgG.Immunochemistry 8:871-874.
Ụkpụrụ echere
HCV-Ab <0.02
A na-atụ aro ka ụlọ nyocha ọ bụla guzobe oke nke ya na-anọchite anya ndị ọrịa ya.
Nsonaazụ ule na ntụgharị asụsụ
- Ihe data a dị n'elu bụ nsonaazụ nke HCV-Ab reagent test, a na-atụkwa aro na ụlọ nyocha ọ bụla kwesịrị ịmepụta ụkpụrụ nchọpụta HCV-Ab dị iche iche maka ndị bi na mpaghara a.Nsonaazụ dị n'elu bụ naanị maka ntụnye aka.
- Nsonaazụ nke usoro a na-adabara naanị na mpaghara ntụaka etinyere na usoro a, ọ nweghịkwa ntụnyere kpọmkwem na ụzọ ndị ọzọ.
- Ihe ndị ọzọ nwekwara ike ịkpata njehie na nsonaazụ nchọpụta, gụnyere ihe kpatara teknụzụ, njehie arụ ọrụ na ihe nlele ndị ọzọ.
Nchekwa na Nkwụsi ike
- Ngwa ahụ bụ ndụ ọnwa 18 site na ụbọchị arụpụtara ya.Chekwaa ngwa ndị a na-ejighị ya na 2-30 Celsius.Emela friji.Ejila ụbọchị ngafe gafere.
- Emepela obere akpa ahụ mechiri emechi ruo mgbe ị dị njikere ịme ule, a na-atụ aro ka iji otu nnwale ahụ n'okpuru ebe achọrọ (okpomọkụ 2-35 ℃, iru mmiri 40-90%) n'ime nkeji 60 ngwa ngwa o kwere mee. .
- A na-eji ihe nlere anya eme ihe ozugbo emepechara.
Ịdọ aka ná ntị na ịkpachara anya
.A ga-emechi ngwa ahụ ma chebe ya pụọ na mmiri.
.A ga-eji usoro ndị ọzọ kwado ihe nlere niile dị mma.
.A ga-ewere ụdịdị niile dị ka ihe nwere ike imetọ.
.Ejila reagenti mebiela.
.Agbanwela reagents n'etiti ngwa nwere nza dị iche iche No.
.Ejighachila kaadị ule na ngwa ngwa ọ bụla enwere ike ijikwa ọzọ.
.Mmejọ, oke ma ọ bụ obere ihe nlele nwere ike ibute nhụsianya nsonaazụ.
LNṅomi
.Dị ka ọ dị na nyocha ọ bụla na-eji ọgwụ mgbochi òké, ohere dị maka nnyonye anya site na mgbochi mgbochi òké mmadụ (HAMA) n'ụdị.Ihe atụ sitere na ndị ọrịa natara ọgwụ mgbochi monoclonal maka nchoputa ma ọ bụ ọgwụgwọ nwere ike ịnwe HAMA.Ụdị ụdị ahụ nwere ike ịkpata nsonaazụ na-adịghị mma ma ọ bụ ụgha ụgha.
Igodo akara akara ejiri:
 | Na Vitro Diagnostic Medical Device |
 | Onye nrụpụta |
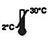 | Na-echekwa na 2-30 ℃ |
 | Ụbọchị mmebi |
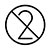 | Ejighachila ya |
 | kpachara anya |
 | Kpọtụrụ ntuziaka maka ojiji |
















